A brain-dead man was attached to a gene-edited pig liver for three days
Doctors are exploring how to use animal organs but keep them outside people's bodies.
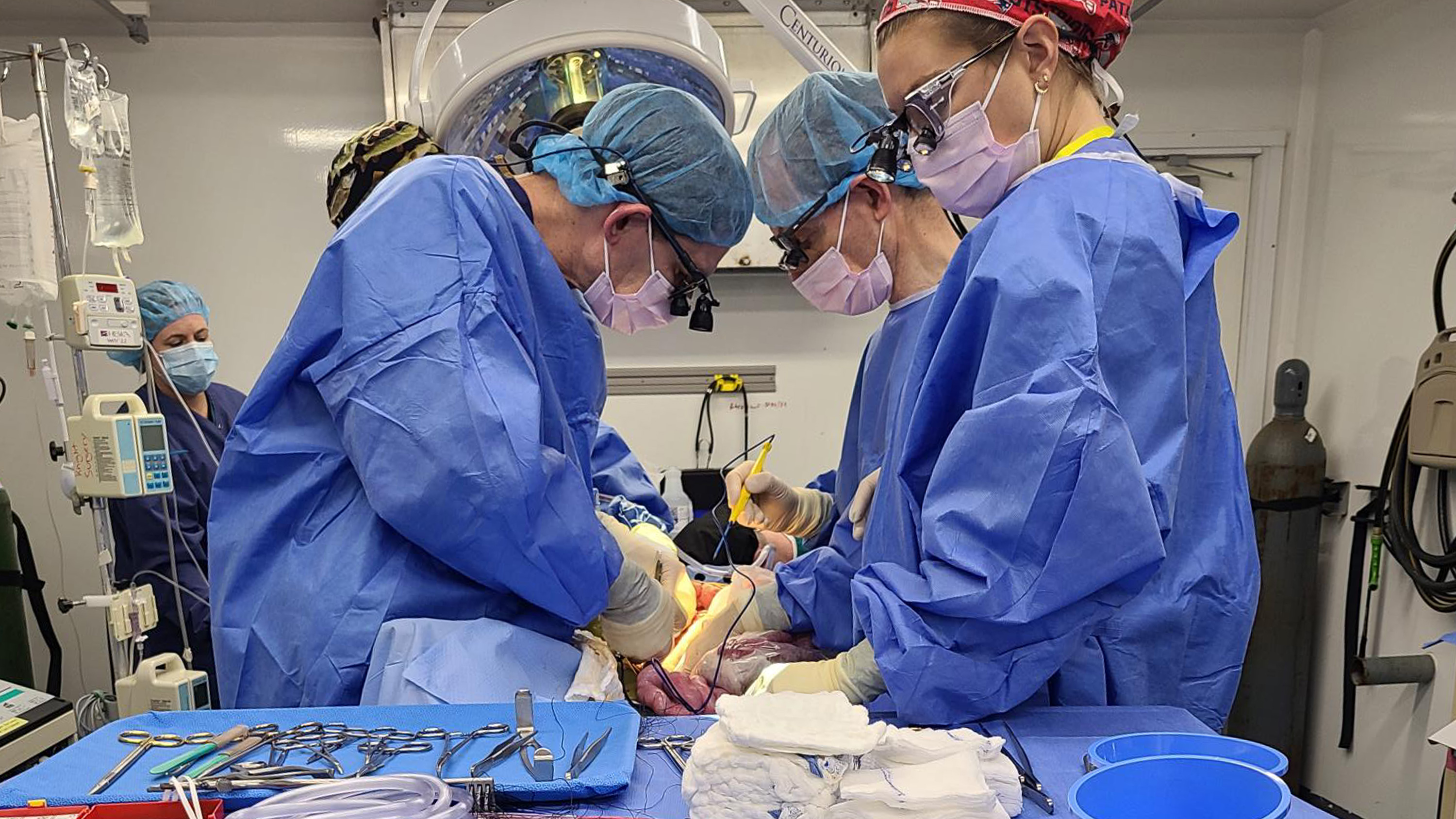
Surgeon Abraham Shaked thinks he has probably carried out more than 2,500 liver transplants. But in December 2023, a team he oversees at the University of Pennsylvania did something he’d never tried before.
Working on the body of a brain-dead man, they attached his veins to a refrigerator-size machine with pig liver mounted in the middle of it.
For three days, the man’s blood passed into the machine, through the pig liver, and back into his body.
This “extracorporeal,” or outside-the-body, liver—whose initial test was announced today by the University of Pennsylvania and a biotech company, eGenesis—is designed to help people survive acute liver failure, which can be caused by infection, poisoning, or (most commonly) too much alcohol.
A damaged liver can’t do its job removing toxins from the body, processing nutrients, and making protein. Hooking people up to an external one could buy them time. “You want to give the liver time to recover … or maintain them until transplant is available,” says Shaked.
The liver test in Philadelphia is also the latest effort to experiment with organs from pigs that have been genetically engineered so their tissues are more compatible with people.
In earlier studies, at the University of Maryland, two men with terminal heart disease had their hearts replaced with hearts from pigs developed by another company, United Therapeutics.
Remarkably, each was able to live with the animal heart, but only for a short time; both died within two months of the transplant. Scientists continue to scrutinize why the hearts failed, but at least the second patient’s heart showed signs of rejection.
Now some doctors say the use of a pig organ that’s kept outside the body might prove easier to pull off, since it only needs to work for a limited time.
“If what we are doing is working in the way that we think it is, I believe this technology will be the first pig organ out there in real clinical use,” says Shaked.
The big goal of pig engineering companies, which include eGenesis, United, and Makana Therapeutics, is to create hearts, kidneys, or lungs that can keep a person alive for years.
To do that, they have all made genetic changes to pigs so that the animal tissue is cloaked from the human immune system, which would otherwise attack the organs.

But using a liver outside the body largely avoids the issue of longer-term organ rejection because it only needs to work for a few days, not years. And the gene edits made to the pigs do seem to protect the organs from severe rejection in the short term. “Here there is no complex immunology,” says Shaked. “We eliminate the rejection question because we don’t use the organ for long. It’s more like a piece of machinery.”
The idea is to use the external organ to support people with liver failure until a human liver transplant becomes available for them or until their livers bounce back, something that’s possible given the organ’s impressive ability to regenerate.
Patients who could benefit include those who overdose on painkillers or who drink too much alcohol over time and develop acute liver failure.
Mike Curtis, CEO of eGenesis, says the biotech company is also testing pig kidneys and hearts that its collaborators have transplanted into baboons. And it hopes to try those organs in humans eventually.
However, starting a little over a year ago, he realized an extracorporeal liver might become a product more quickly. “People were like, ‘We have to do a transplant, we have got to do a transplant.’ But we also have to prove there is something investible here, and liver is an acute need with few competing products,” says Curtis. “It’s not a transplant, and it’s a little weird, but the pieces kept falling together.”
For biotech companies, it’s crucial to get a product into human testing as soon as they can, as that’s when big drug company partners come knocking. And pig organ transplants have always had a reputation as a speculative technology that’s never quite succeeded.
“There is always curiosity. Everyone takes a meeting,” says Curtis. “They do view it as the future, but is it next year, or 100 years from now?”
“With a heart transplant, you are really swinging for the fence,” he says. “Whereas with extracorporeal, it’s a little more like how products usually get developed. You can try for steady improvements.”
This is the first time an organ from one of the eGenesis pigs has been tried with a human, but Curtis says eGenesis is ready to apply for permission to start a formal trial of the liver system this year. If it gets the green light, that could make it the first formal clinical trial of any gene-edited pig organ. (The heart transplants were considered one-off bids to help patients, not a clinical trial.)
The experiment started on December 22, after the family of a elderly man who’d suffered a brain bleed agreed to let his body be used in the research. He was brain dead, but his heart was still beating.
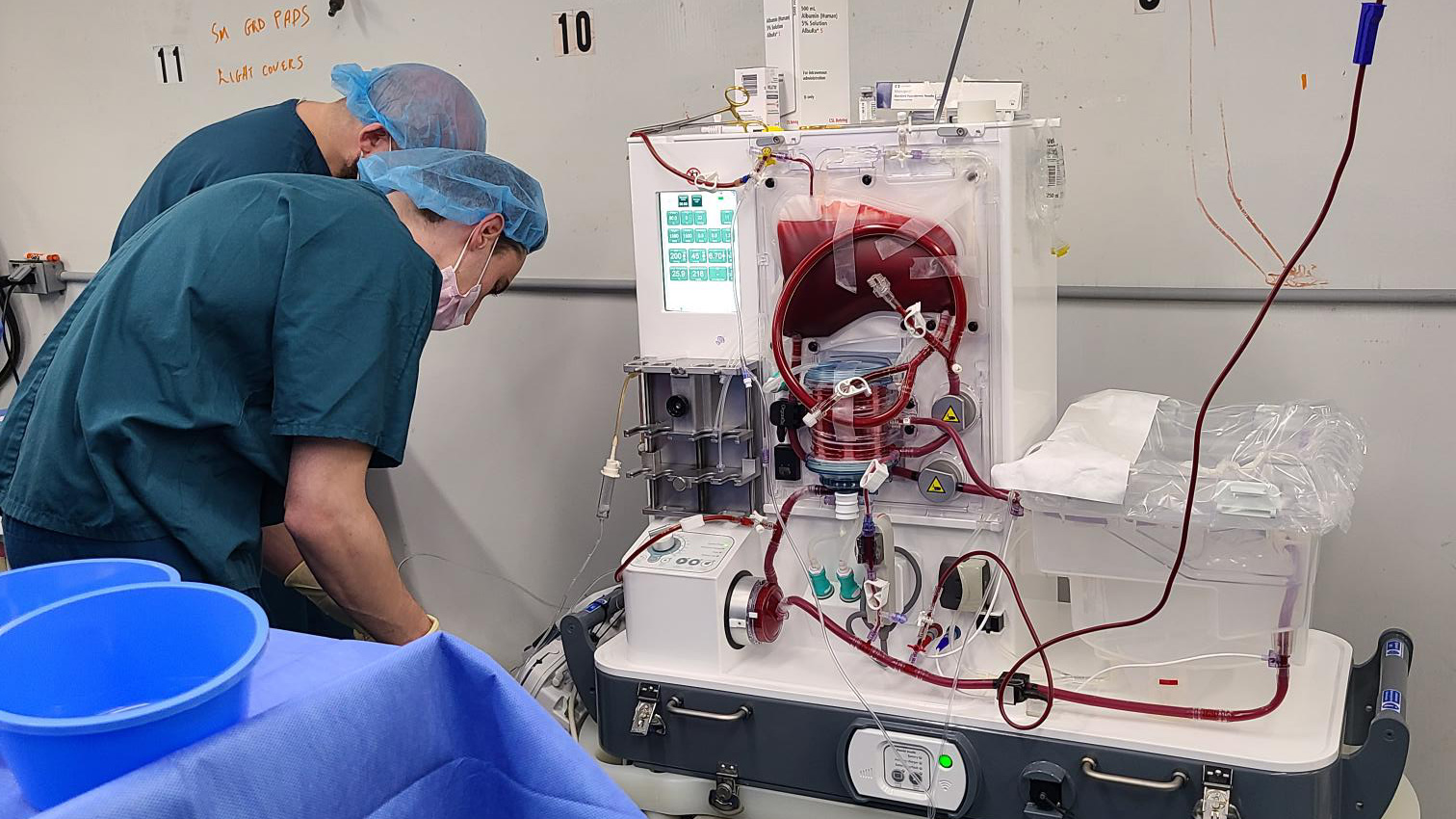
During the experiment, the pig liver was mounted into a device from the company OrganOx that is normally used to keep donated human organs warm and perfused with blood so they will be available for transplant longer.
In this case, tubes connected to the subject’s veins were routed into the machine, and the two remained attached for 72 hours.
The idea of extracorporeal organs has been tried before. In the 1990s, researchers connected several patients to livers taken from ordinary pigs, but the organs quickly deteriorated. According to eGenesis, its liver, which came from a genetically modified Yucatan minipig, was still healthy even after three days.
Unlike hearts and kidneys, pig livers probably aren’t plausible candidates for transplanting directly into a person. One of the organ’s jobs is to mass-produce proteins, fats, and glucose—and the pig versions of those molecules would probably provoke a powerful immune reaction against even a genetically modified liver.
Adam Griesemer, a surgical director for the NYU Langone Transplant Institute, says extracorporeal use is “probably the only application” for pig livers.
Deep Dive
Biotechnology and health
How scientists traced a mysterious covid case back to six toilets
When wastewater surveillance turns into a hunt for a single infected individual, the ethics get tricky.
An AI-driven “factory of drugs” claims to have hit a big milestone
Insilico is part of a wave of companies betting on AI as the "next amazing revolution" in biology
The quest to legitimize longevity medicine
Longevity clinics offer a mix of services that largely cater to the wealthy. Now there’s a push to establish their work as a credible medical field.
There is a new most expensive drug in the world. Price tag: $4.25 million
But will the latest gene therapy suffer the curse of the costliest drug?
Stay connected
Get the latest updates from
MIT Technology Review
Discover special offers, top stories, upcoming events, and more.