The secret to a new drug could be hiding in your genes
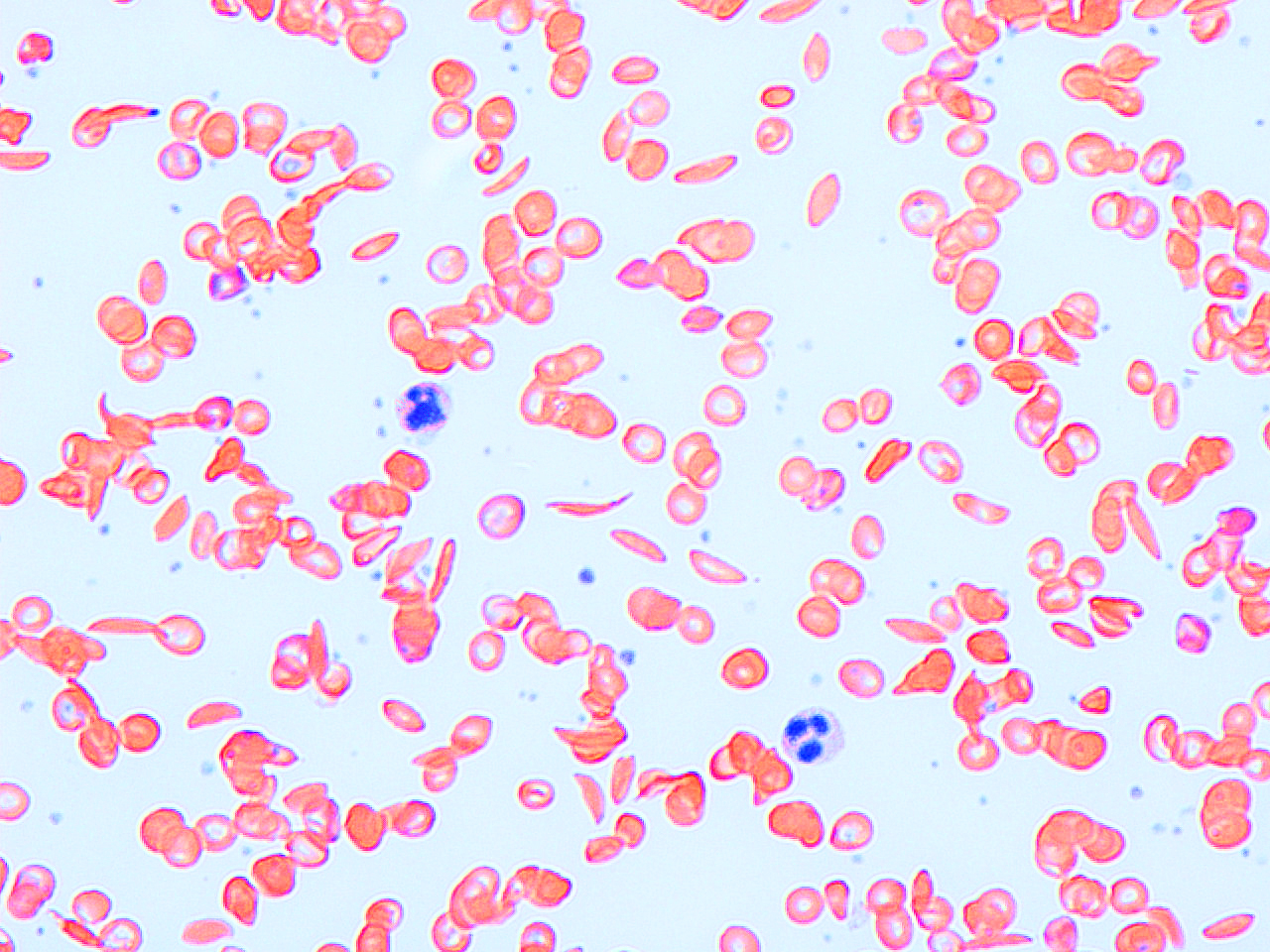
Sickle-cell disease is caused by a one-letter spelling mistake in the hemoglobin gene. But not everyone who inherits the error suffers the worst effects of the blood disease.
By 2008, scientists had figured out why. It’s because some of them have a second genetic peculiarity that causes their bodies to continue to manufacture fetal hemoglobin, a version of the oxygen-carrying substance that’s normally made only until birth.
The second genetic change acts as a modifier—in effect, a genetic countermeasure against disease.
Now, a startup called Maze Therapeutics has amassed an impressive bank account ($191 million from investors Third Rock Ventures, ARCH Venture Partners, and others) to carry out a search for such modifiers and create drugs that mimic their effects.
The startup—which says it wants to find out why some people get sick and others don’t—plans to mine public genetic databases to locate people with gene errors who’ve not had serious medical problems.
The hunt for such genetic exceptions is one of the latest fads in drug development. It got attention in 2016 after the so-called “Resilience Project” said it had found 13 people who should have had serious childhood diseases, but didn’t. Other companies, including Regeneron Pharmaceuticals, have also staked their futures on assembling private troves of DNA data and looking for outliers.
Notably, Maze says it won’t need to create its own genetic database, a costly undertaking. Instead, it will use free public resources, including a British biobank of 500,000 people’s genes and medical records. Soon, similar data could become available from the US Department of Veterans Affairs. The company’s founders include Mark Daly, who oversees a genetics institute in Finland, a country building its own biobank.
“There is a remarkable amount of genetic data out there,” says Charles Homcy, who is CEO of Maze. “Five years ago it would have been a mistake to try this.”
New tools also exist to search for modifiers in the lab. One of the company’s other scientific founders, Jonathan Weissman, a researcher at the University of California, San Francisco, says it’s possible to take thousands of human cells afflicted with a mutation (such as the one causing sickle-cell) and, using the gene-editing tool CRISPR, introduce a different genetic modification into each.
Then, employing novel techniques for measuring single cells, researchers can see whether any of the modifications counteracted the disease. “You take a cell with a disease-causing gene and then see if you can turn it back to normal,” says Weissman. “We can do 100,000 experiments at once because each cell is its own experiment.”
There are already examples of drugs based on the discovery of modifier genes. One treatment, called Spinraza, has been successfully treating spinal muscular atrophy, an often fatal childhood disease.
Deep Dive
Biotechnology and health
How scientists traced a mysterious covid case back to six toilets
When wastewater surveillance turns into a hunt for a single infected individual, the ethics get tricky.
An AI-driven “factory of drugs” claims to have hit a big milestone
Insilico is part of a wave of companies betting on AI as the "next amazing revolution" in biology
The quest to legitimize longevity medicine
Longevity clinics offer a mix of services that largely cater to the wealthy. Now there’s a push to establish their work as a credible medical field.
There is a new most expensive drug in the world. Price tag: $4.25 million
But will the latest gene therapy suffer the curse of the costliest drug?
Stay connected
Get the latest updates from
MIT Technology Review
Discover special offers, top stories, upcoming events, and more.