The coronavirus test that might exempt you from social distancing—if you pass
On Monday, President Trump announced that the US had tested over a million patient samples for coronavirus, by far more than any other country in the world. Though the horrendously slow rollout of testing has already set America back in its effort to stop the spread of covid-19, testing is still vital. To beat the virus and stop its spread, says the World Health Organization, we need to identify those who are infected and isolate them, as well as those at risk (who ought to be self-isolating too, whether they are symptomatic or asymptomatic). We also need to figure out which communities can expect to see a rise in coronavirus cases, and where to allocate resources in anticipation of rising hospitalizations.
But there’s also a serious need for us to find out who has already been infected and is now, presumably, immune to the virus (at least for a while). Since the coronavirus outbreak began, many different groups have ramped up their efforts to develop a serological test that looks for antibodies to the virus—an indication of whether an individual was once infected. Should a test like this ever become available to the public, it could radically shape how we decide who gets to leave home and return to some semblance of normal life.
More on coronavirus
Our most essential coverage of covid-19 is free, including:
How does the coronavirus work?
What are the potential treatments?
What's the right way to do social distancing?
Other frequently asked questions about coronavirus
---
Newsletter: Coronavirus Tech Report
Zoom show: Radio Corona
See also:
Please click here to subscribe and support our non-profit journalism.
Here are the biggest things you should know about the status of antibody testing for covid-19.
Why do we want antibody testing?
Many infected individuals experience only mild or moderate symptoms that clear out fairly quickly. Since there are simply not enough test kits to go around, a lot of people who aren’t showing more severe symptoms are being turned away for testing. Those individuals (myself included) are effectively in limbo, having no way to verify if they were once sick and now potentially immune, or still at risk of being sick and spreading the virus. Moreover, if we’re not able to test everyone, we have no way to really answer questions such as how widespread the infection is, what the true fatality rate is, and what kinds of measures to stop the spread are actually working.
Antibody testing that’s made available en masse might be able to help answer some of those basic questions. Once we have a better understanding of how immunity works with the coronavirus, it could also give survivors of the infection confirmation that they are now immune, meaning they no longer pose a threat to others and could potentially return to work and public life. This would be especially critical for clinics and hospitals experiencing staffing shortages, or infrastructure and utilities providers who need properly trained workers to keep things like our power grids running.
How does it work?
When the body is introduced to a pathogen, the immune system develops tailor-made antibodies that act against the infection. Antibodies can last a long time—anywhere from a couple of years to a lifetime, depending on the disease. During the period that immunity lasts, your body is prepared to ramp up production of those antibodies to neutralize the threat should it ever appear again.
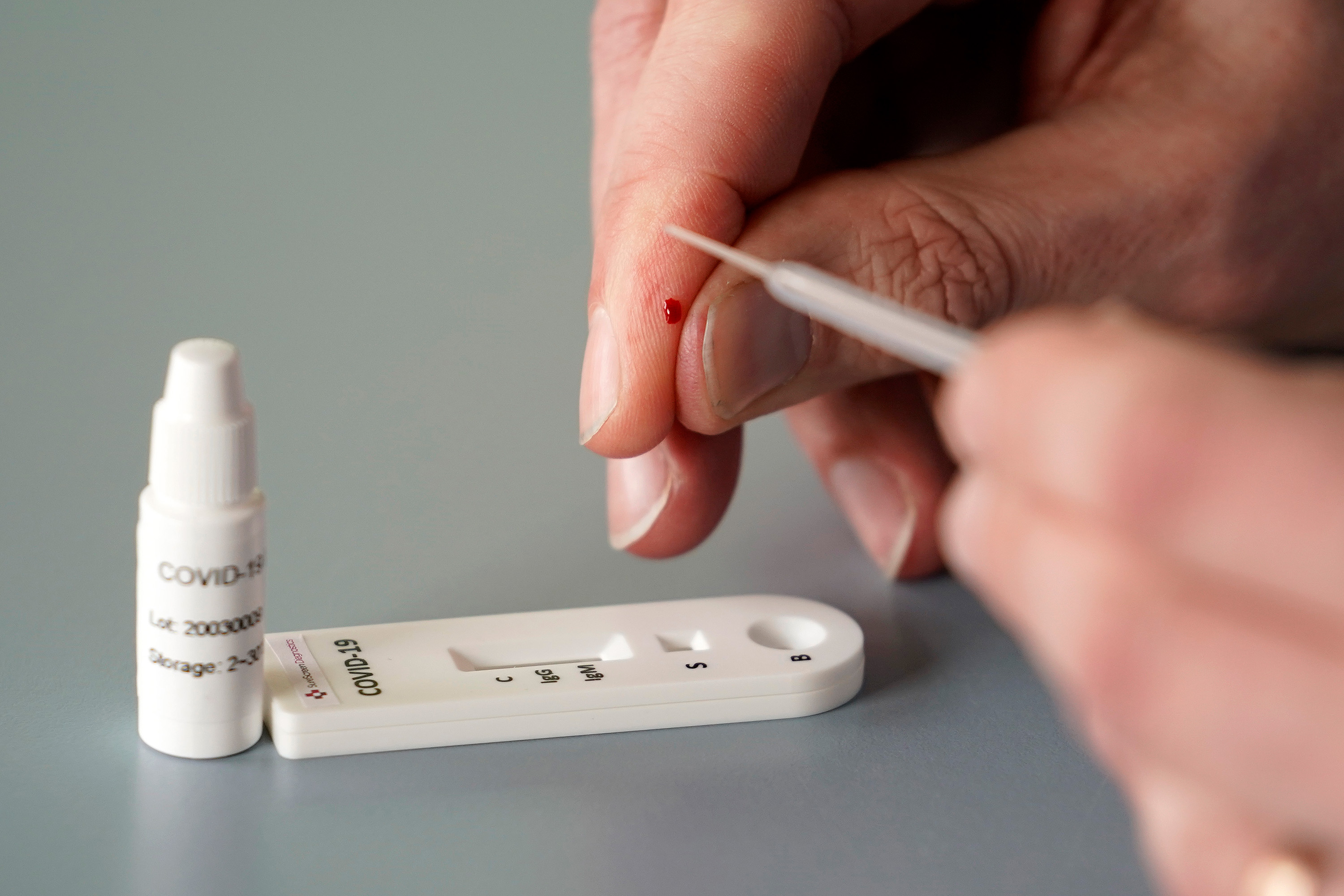
An antibody test, also known as a serology test, analyzes a patient’s serum—the liquid portion of blood that excludes cells and clotting factors but includes antibodies. Many of these tests are simple and require only a small sample, like a finger prick. In this case, through a technique like ELISA (enzyme-linked immunosorbent assay), clinicians look for antibodies that were made in response to the large protein that sticks out of the coronavirus’s surface. A viral fragment is placed on a plate. If there’s an antibody in the patient sample, it will attach to this “spike” protein. Another antibody, engineered by the clinicians and capable of attaching to the first antibody, is introduced to the solution. When they bind, the new antibody will activate an enzyme that changes the color of the solution, indicating that the patient has the antibodies we’re looking for, and has therefore been exposed to the coronavirus.
How is this different from the testing we already do?
The way we’re testing infections right now is by looking for viral genetic material in patient samples. Using a method called polymerase chain reaction (PCR), clinicians can amplify any coronavirus RNA in a patient’s nasal swab so its presence can be confirmed. Viral DNA or RNA can be found in the body as soon as an infection begins, even if you’re asymptomatic. But it disappears soon after the immune system clears the infection out. So this type of test is useful to find out who is currently infected, but not who once was infected.
Antibodies, on the other hand, aren’t developed until several days after infection has taken hold, so they aren’t a useful indicator of who is currently infected. But because they’re around in the blood in large numbers for many months after infection, they would be extremely useful to identify past cases long after the infection has been beaten.
How much does it cost?
A serological test for coronavirus antibodies is much less costly than a PCR test that looks for coronavirus genetic material. California-based Biomerica, for example, sells a serological test for less than $10. A PCR test for covid-19 can cost up to $51 under Medicare.
How fast is it?
You can get results from a serological test in just minutes. Many groups are working on versions that can be run at home, with no need to send samples to a lab. A PCR test takes hours to run, and because samples must typically be shipped back and forth from the testing site, patients usually don’t get results for at least several days (although the FDA is fast-tracking a portable point-of-care genetic test for coronavirus that’s supposed to take less than 15 minutes).
Who’s working on this?
Many, many groups. Singapore, China, and other countries have already conducted limited numbers of antibody testing. A group led by virologist Florian Krammer at the Icahn School of Medicine at Mount Sinai in New York City recently developed an ELISA-based antibody test for covid-19. American companies like Biomerica and Chembio Diagnostics (from New York) are selling antibody tests outside the US, with aggressive plans to get these kits up to snuff for FDA approval. BioMedomics of North Carolina, in collaboration with medical tech company BD, just launched a point-of-care test that can be administered at the doctor’s office and give results in 15 minutes. The UK has its own test, developed by Public Health England, and recently ordered 3.5 million kits to be distributed by Amazon and pharmacies around the country in just a matter of days.
What are the limitations?
An antibody test will show that someone is immune, but it doesn’t guarantee that the person is no longer contagious. A PCR test might be necessary to show whether or not the body is still battling the coronavirus and still infectious to others. In other words, you’d want to test positive for immunity through an antibody test, and negative for the virus through a PCR test.
There is a huge concern about the accuracy of serological tests. PCR testing, for all its drawbacks, is still considered pretty accurate. In an antibody test, however, a patient might test positive for covid-19 because of antibodies against a different coronavirus (like ones that cause the common cold). Two patients might be infected and recover at about the same time, but one’s antibody test might not stay positive as long as the other’s. And again, there is a huge window of uncertainty because it could take up to a week for a body to start generating antibodies against the virus after infection has set in. Taking the test during infection may not deliver a very confident result. An accuracy of, say, 80% still leaves one in five people with a false result. Spain recently recalled more than 8,000 Chinese-made test kits because of worries about inaccurate results. More than a dozen companies that have notified the FDA they are producing antibody tests are allowed to begin distributing the tests to hospitals and doctors’ offices, but they must carry disclaimer statements that read: “This test has not been reviewed by the FDA.” Accuracy and reliability won’t be ensured without validation and experience over time.
And because covid-19 is such a new disease, we don’t know how long immunity will last. Right now the virus seems to be mutating slowly and shouldn’t pose an annual problem like the flu, but we’ve only been studying it for a little over three months. Tony Mazzulli, chief microbiologist with Toronto's Sinai Health, told the New York Times it’s also unclear whether antibodies would prevent infection from exposure to a large amount of the virus, as in a hospital setting.
Deep Dive
Biotechnology and health
How scientists traced a mysterious covid case back to six toilets
When wastewater surveillance turns into a hunt for a single infected individual, the ethics get tricky.
An AI-driven “factory of drugs” claims to have hit a big milestone
Insilico is part of a wave of companies betting on AI as the "next amazing revolution" in biology
The quest to legitimize longevity medicine
Longevity clinics offer a mix of services that largely cater to the wealthy. Now there’s a push to establish their work as a credible medical field.
There is a new most expensive drug in the world. Price tag: $4.25 million
But will the latest gene therapy suffer the curse of the costliest drug?
Stay connected
Get the latest updates from
MIT Technology Review
Discover special offers, top stories, upcoming events, and more.