Eugenics 2.0: We’re at the Dawn of Choosing Embryos by Health, Height, and More
Nathan Treff was diagnosed with type 1 diabetes at 24. It’s a disease that runs in families, but it has complex causes. More than one gene is involved. And the environment plays a role too.
So you don’t know who will get it. Treff’s grandfather had it, and lost a leg. But Treff’s three young kids are fine, so far. He’s crossing his fingers they won’t develop it later.
Now Treff, an in vitro fertilization specialist, is working on a radical way to change the odds. Using a combination of computer models and DNA tests, the startup company he’s working with, Genomic Prediction, thinks it has a way of predicting which IVF embryos in a laboratory dish would be most likely to develop type 1 diabetes or other complex diseases. Armed with such statistical scorecards, doctors and parents could huddle and choose to avoid embryos with failing grades.
IVF clinics already test the DNA of embryos to spot rare diseases, like cystic fibrosis, caused by defects in a single gene. But these “preimplantation” tests are poised for a dramatic leap forward as it becomes possible to peer more deeply at an embryo’s genome and create broad statistical forecasts about the person it would become.
The advance is occurring, say scientists, thanks to a growing flood of genetic data collected from large population studies. As statistical models known as predictors gobble up DNA and health information about hundreds of thousands of people, they’re getting more accurate at spotting the genetic patterns that foreshadow disease risk. But they have a controversial side, since the same techniques can be used to project the eventual height, weight, skin tone, and even intelligence of an IVF embryo.
In addition to Treff, who is the company’s chief scientific officer, the founders of Genomic Prediction are Stephen Hsu, a physicist who is vice president for research at Michigan State University, and Laurent Tellier, a Danish bioinformatician who is CEO. Both Hsu and Tellier have been closely involved with a project in China that aims to sequence the genomes of mathematical geniuses, hoping to shed light on the genetic basis of IQ.
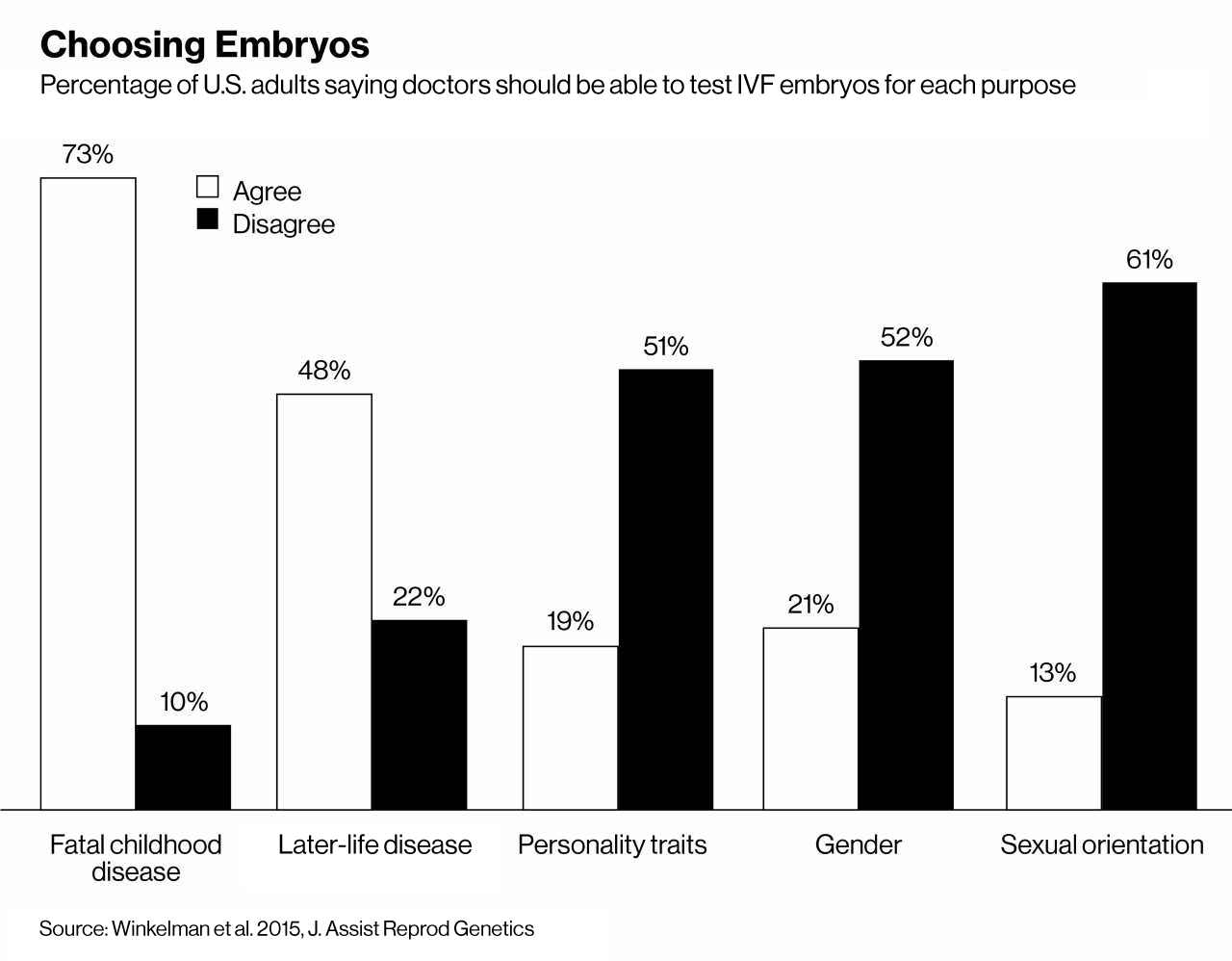
Spotting outliers
The company’s plans rely on a tidal wave of new knowledge showing how small genetic differences can add up to put one person, but not another, at high odds for diabetes, a neurotic personality, or a taller or shorter height. Already, such “polygenic risk scores” are used in direct-to-consumer gene tests, such as reports from 23andMe that tell customers their genetic chance of being overweight.
For adults, risk scores are little more than a novelty or a source of health advice they can ignore. But if the same information is generated about an embryo, it could lead to existential consequences: who will be born, and who stays in a laboratory freezer.
“I remind my partners, ‘You know, if my parents had this test, I wouldn’t be here,’” says Treff, a prize-winning expert on diagnostic technology who is the author of more than 90 scientific papers.
Genomic Prediction was founded this year and has raised funds from venture capitalists in Silicon Valley, though it declines to say who they are. Tellier, whose inspiration is the science fiction film Gattaca, says the company plans to offer reports to IVF doctors and parents identifying “outliers”—those embryos whose genetic scores put them at the wrong end of a statistical curve for disorders such as diabetes, late-life osteoporosis, schizophrenia, and dwarfism, depending on whether models for those problems prove accurate.
The company’s concept, which it calls expanded preimplantation genetic testing, or ePGT, would effectively add a range of common disease risks to the menu of rare ones already available, which it also plans to test for. Its promotional material uses a picture of a mostly submerged iceberg to get the idea across. “We believe it will become a standard part of the IVF process,” says Tellier, just as a test for Down syndrome is a standard part of pregnancy.
Some experts contacted by MIT Technology Review said they believed it’s premature to introduce polygenic scoring technology into IVF clinics—though perhaps not by very much. Matthew Rabinowitz, CEO of the prenatal-testing company Natera, based in California, says he thinks predictions obtained today could be “largely misleading” because DNA models don’t function well enough. But Rabinowitz agrees that the technology is coming.
“You are not going to stop the modeling in genetics, and you are not going to stop people from accessing it,” he says. “It’s going to get better and better.”
Sharp questions
Testing embryos for disease risks, including risks for diseases that develop only late in life, is considered ethically acceptable by U.S. fertility doctors. But the new DNA scoring models mean parents might be able to choose their kids on the basis of traits like IQ or adult weight. That’s because, just like type 1 diabetes, these traits are the result of complex genetic influences the predictor algorithms are designed to find.
The model predicted people’s height from their DNA data to within three or four centimeters.
“It’s the camel’s nose under the tent. Because if you are doing it for something more serious, then it’s trivially easy to look for anything else,” says Michelle Meyer, a bioethicist at the Geisinger Health System who analyzes issues in reproductive genetics. “Here is the genomic dossier on each embryo. And you flip through the book.” Imagine picking the embryo most likely to get into Harvard like Mom, or to be tall like Dad.
For Genomic Prediction, a tiny startup based out of a tech incubator in New Jersey, such questions will be especially sharply drawn. That is because of Hsu’s long-standing interest in genetic selection for superior intelligence.
In 2014, Hsu authored an essay titled “Super-Intelligent Humans Are Coming,” in which he argued that selecting embryos for intelligence could boost the resulting child’s IQ by 15 points.
Genomic Prediction says it will only report diseases—that is, identify those embryos it thinks would develop into people with serious medical problems. Even so, on his blog and in public statements, Hsu has for years been developing a vision that goes far beyond that.
“Suppose I could tell you embryo four is going to be the tallest, embryo three is going to be the smartest, embryo two is going to be very antisocial. Suppose that level of granularity was available in the reports,” he told the conservative radio and YouTube personality Stefan Molyneux this spring. “That is the near-term future that we as a civilization face. This is going to be here.”

Measuring height
The fuel for the predictive models is a deluge of new data, most recently genetic readouts and medical records for 500,000 middle-aged Britons that were released in July by the U.K. Biobank, a national precision-medicine project in that country.
The data trove included, for each volunteer, a map of about 800,000 single-nucleotide polymorphisms, or SNPs—points where their DNA differs slightly from another person’s. The release caused a pell-mell rush by geneticists to update their calculations about exactly how much of human disease, or even routine behaviors like bread consumption, these genetic differences could explain.
Armed with the U.K. data, Hsu and Tellier claimed a breakthrough. For one easily measured trait, height, they used machine-learning techniques to create a predictor that behaved flawlessly. They reported that the model could, for the most part, predict people’s height from their DNA data to within three or four centimeters.
Height is currently the easiest trait to predict. It’s determined mostly by genes, and it’s always recorded in population databases. But Tellier says genetic databases are “rapidly approaching” the size needed to make accurate predictions about other human features, including risk for diseases whose true causes aren’t even known.
Tellier says Genomic Prediction will zero in on disease traits for which the predictors already perform fairly well, or will soon. Those include autoimmune disorders like the illness Treff suffers from. In those conditions, a smaller set of genes dominates the predictions, sometimes making them more reliable.
A report from Germany in 2014, for instance, found it was possible to distinguish fairly accurately, from a polygenic DNA score alone, between a person with type 1 diabetes and a person without it. While the scores aren’t perfectly accurate, consider how they might influence a prospective parent. On average, children of a man with type 1 diabetes have a one in 17 chance of developing the ailment. Picking the best of several embryos made in an IVF clinic, even with an error-prone predictor, could lower the odds.
In the case of height, Genomic Prediction hopes to use the model to help identify embryos that would grow into adults shorter than 4'10", the medical definition of dwarfism, says Tellier. There are many physical and psychological disadvantages to being so short. Eventually the company could also have the ability to identify intellectual problems, such as embryos with a predicted IQ of less than 70.
The company doesn’t intend to give out raw trait scores to parents, only to flag embryos likely to be abnormal. That is because the product has to be “ethically defensible,” says Hsu: “We would only reveal the negative outlier state. We don’t report, ‘This guy is going to be in the NBA.’”
Some scientists doubt the scores will prove useful at picking better people from IVF dishes. Even if they’re accurate on the average, for individuals there’s no guarantee of pinpoint precision. What’s more, environment has as big an impact on most traits as genes do. “There is a high probability that you will get it wrong—that would be my concern,” says Manuel Rivas, a professor at Stanford University who studies the genetics of Crohn’s disease. “If someone is using that information to make decisions about embryos, I don’t know what to make of it.”
“We’ve seen such a crazy change in the number of people we are able to study.”
Efforts to introduce this type of statistical scoring into reproduction have, in the past, drawn criticism. In 2013, 23andMe provoked outrage when it won a patent on the idea of drop-down menus parents could use to pick sperm or egg donors—say, to try to get a specific eye color. The company, funded by Google, quickly backpedaled.
But since then, polygenic scores have become a routine aspect of novelty DNA tests. A company called HumanCode sells a $199 test online that uses SNP scores to tell two people about how tall their kids might be. In the dairy cattle industry, polygenic tests are widely used to rate young animals for how much milk they’ll produce.
“At a broad level, our understanding of complex traits has evolved. It’s not that there are a few genes contributing to complex traits; it’s tens, or thousands, or even all genes,” says Meyer, the Geisinger professor. “That has led to polygenic risk scores. It’s many variants, each with small contributions of their own, but which have a significant contribution together. You add them up.” In his predictor for height, Hsu eventually made use of 20,000 variants to guess how tall each person was.
Measuring embryos
Around the world, a million couples undergo IVF each year; in the U.S., test-tube babies account for 1 percent of births. Preimplantation genetic diagnosis, or PGD, has been part of the technology since the 1990s. In that procedure, a few cells are plucked from a days-old embryo growing in a laboratory so they can be tested.
Until now, doctors have used PGD to detect embryos with major abnormalities, such as missing chromosomes, as well as those with “single gene” defects. Parents who carry the defective gene that causes Huntington’s disease, for instance, can use embryo tests to avoid having a child with the fatal brain ailment.

The obstacle to polygenic tests has been that with so few cells, it’s been difficult to get the broad, accurate view of an embryo’s genome necessary to perform the needed calculations. “It’s very hard to make reliable measurements on that little DNA,” says Rabinowitz, the Natera CEO.
Tellier says Genomic Prediction has developed an improved method for analyzing embryonic DNA, which he says will first be used to improve on traditional PGD, combing many single-gene tests into one. Tellier says the same technique is what will permit it to collect polygenic scores on embryos, although the company did not describe the method in detail. But other scientists have already demonstrated ways to overcome the accuracy barrier.
In 2015, a team led by Rabinowitz and Jay Shendure of the University of Washington did it by sequencing in detail the genomes of two parents undergoing IVF. That let them infer the embryo’s genome sequence, even though the embryo test itself was no more accurate than before. When the babies were born, they found they’d been right.
“We do have the technology to reconstruct the genome of an embryo and create a polygenic model,” says Rabinowitz, whose publicly traded company is worth about $600 million, and who says he has been mulling whether to enter the embryo-scoring business. “The problem is that the models have not quite been ready for prime time.”
That’s because despite Hsu’s success with height, the scoring algorithms have significant limitations. One is that they’re built using data mostly from Northern Europeans. That means they may not be useful for people from Asia or Africa, where the pattern of SNPs is different, or for people of mixed ancestry. Even their performance for specific families of European background can’t be taken for granted unless the procedure is carefully tested in a clinical study, something that’s never been done, says Akash Kumar, a Stanford resident physician who was lead author of the Natera study.
Kumar, who treats young patients with rare disorders, says the genetic predictors raise some “big issues.” One is that the sheer amount of genetic data becoming available could make it temptingly easy to assess nonmedical traits. “We’ve seen such a crazy change in the number of people we are able to study,” he says. “Not many have schizophrenia, but they all have a height and a body-mass index. So the number of people you can use to build the trait models is much larger. It’s a very unique place to be, thinking what we should do with this technology.”
Smarter kids
This week, Genomic Prediction manned a booth at the annual meeting of the American Society for Reproductive Medicine. That organization, which represents fertility doctors and scientists, has previously said it thinks testing embryos for late-life conditions, like Alzheimer’s, would be “ethically justified.” It cited, among other reasons, the “reproductive liberty” of parents.
“There will be some future debate over whether this should be legal, or made illegal. Countries will have referendums on it.”
The society has been more ambivalent about choosing the sex of embryos (something that conventional PGD allows), leaving it to the discretion of doctors. Combined, the society’s positions seem to open the door to any kind of measurement, perhaps so long as the test is justified for a medical reason.
Hsu has previously said he thinks intelligence is “the most interesting phenotype,” or trait, of all. But when he tried his predictor to see what it could say about how far along in school the 500,000 British subjects from the U.K. Biobank had gotten (years of schooling is a proxy for IQ), he found that DNA couldn’t predict it nearly as well as it could predict height.
Yet DNA did explain some of the difference. Daniel Benjamin, a geno-economist at the University of Southern California, says that for large populations, gene scores are already as predictive of educational attainment as whether someone grew up in a rich or poor family. He adds that the accuracy of the scores has been steadily improving. Scoring embryos for high IQ, however, would be “premature” and “ethically contentious,” he says.
Hsu’s prediction is that “billionaires and Silicon Valley types” will be the early adopters of embryo selection technology, becoming among the first “to do IVF even though they don’t need IVF.” As they start producing fewer unhealthy children, and more exceptional ones, the rest of society could follow suit.
“I fully predict it will be possible,” says Hsu of selecting embryos with higher IQ scores. “But we’ve said that we as a company are not going to do it. It’s a difficult issue, like nuclear weapons or gene editing. There will be some future debate over whether this should be legal, or made illegal. Countries will have referendums on it.”
Deep Dive
Biotechnology and health
How scientists traced a mysterious covid case back to six toilets
When wastewater surveillance turns into a hunt for a single infected individual, the ethics get tricky.
An AI-driven “factory of drugs” claims to have hit a big milestone
Insilico is part of a wave of companies betting on AI as the "next amazing revolution" in biology
The quest to legitimize longevity medicine
Longevity clinics offer a mix of services that largely cater to the wealthy. Now there’s a push to establish their work as a credible medical field.
There is a new most expensive drug in the world. Price tag: $4.25 million
But will the latest gene therapy suffer the curse of the costliest drug?
Stay connected
Get the latest updates from
MIT Technology Review
Discover special offers, top stories, upcoming events, and more.