Tiny faux organs could crack the mystery of menstruation
Researchers are using organoids to unlock one of the human body’s most mysterious—and miraculous—processes.
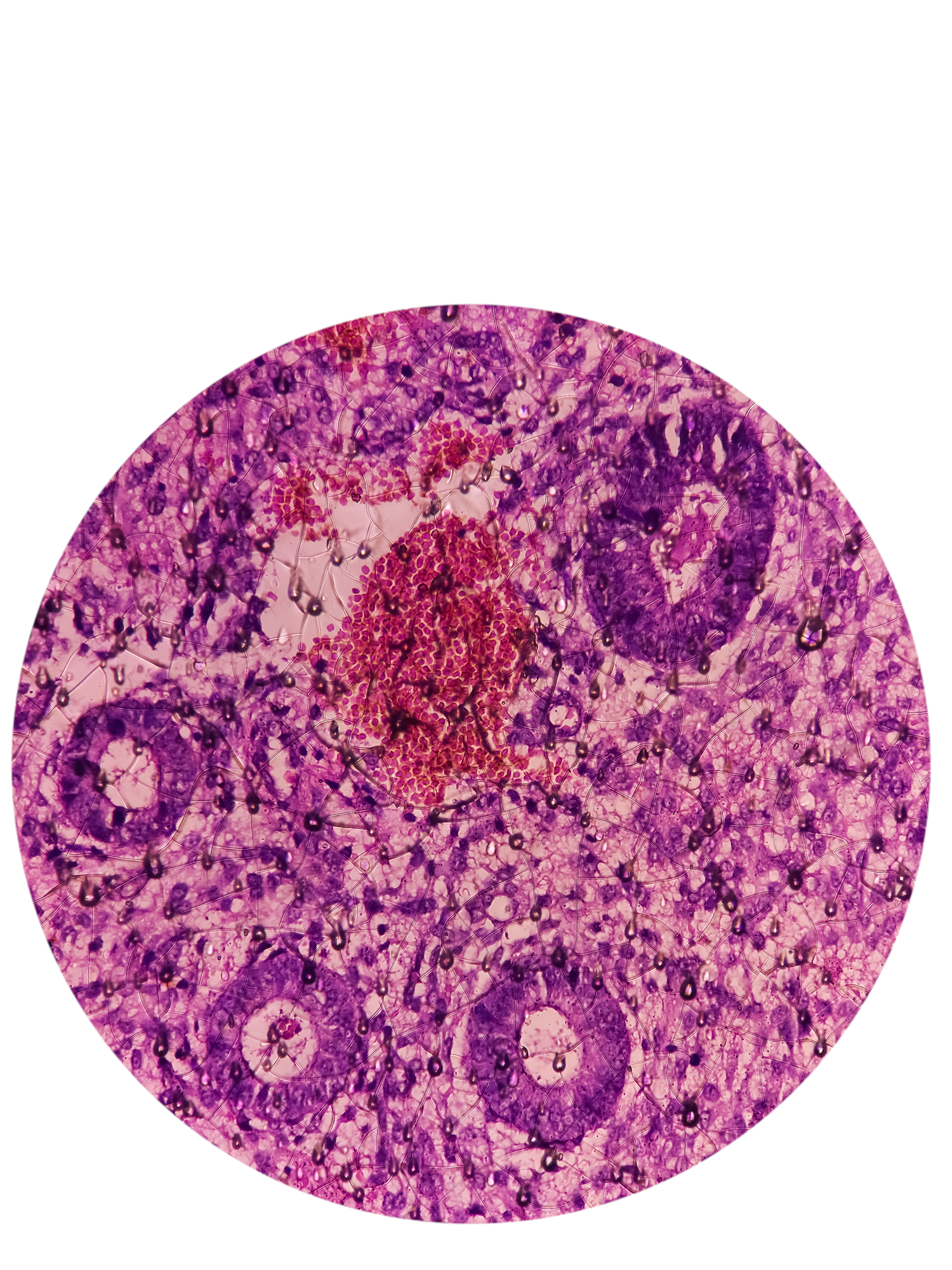
In the center of the laboratory dish, there was a subtle white film that could only be seen when the light hit the right way. Ayse Nihan Kilinc, a reproductive biologist, popped the dish under the microscope, and an image appeared on the attached screen. As she focused the microscope, the film resolved into clusters of droplet-like spheres with translucent interiors and thin black boundaries. In this magnified view, the structures ranged in size from as small as a quarter to as large as a golf ball. In reality, each was only as big as a few grains of sand.
“They’re growing,” Kilinc said, observing that their plump shapes were a promising sign. “These are good organoids.”
Kilinc, who works in the lab of biological engineer Linda Griffith at MIT, is among a small group of scientists using new tools akin to miniature organs to study a poorly understood—and frequently problematic—part of human physiology: menstruation. Heavy, sometimes debilitating periods strike at least a third of people who menstruate at some point in their lives, causing some to miss weeks of work or school every year and jeopardizing their professional standing. Anemia threatens about two-thirds of people with heavy periods. And when menstrual blood flows through the fallopian tubes and into the body cavity, it’s thought to sometimes create painful lesions—characteristics of a disease called endometriosis, which can require multiple surgeries to control.
No one is entirely sure how—or why—the human body choreographs this monthly dance of cellular birth, maturation, and death. Many people desperately need treatments to make their period more manageable, but it’s difficult for scientists to design medications without understanding how menstruation really works.
That understanding could be in the works, thanks to endometrial organoids—biomedical tools made from bits of the tissue that lines the uterus, called the endometrium. To make endometrial organoids, scientists collect cells from a human volunteer and let those cells self-organize in laboratory dishes, where they develop into miniature versions of the tissue they came from. The research is still very much in its infancy. But organoids have already provided insights into how endometrial cells communicate and coordinate, and why menstruation is routine for some people and fraught for others. Some researchers are hopeful that these early results mark the dawn of a new era. “I think it’s going to revolutionize the way we think about reproductive health,” says Juan Gnecco, a reproductive engineer at Tufts University.
An uncommon problem
Periods are rare in the animal kingdom. The human body goes through the menstrual cycle to prepare the uterus to welcome a fetus, whether one is likely to show up or not. In contrast, most animals prepare the uterus only once a fetus is already present.
That cycle is a constant pattern of wounding and repair. The process starts when levels of a hormone called progesterone plummet, indicating that no baby will be growing in the uterus that month. Removing progesterone triggers a response similar to what happens when the body fights off an infection. Inflammation injures the endometrium. Over the next five or so days, the damaged tissue sloughs off and flows out of the body.
As soon as the bleeding starts, the endometrium begins to heal. Over the course of about 10 days, this tissue quadruples in thickness. No other human tissue is known to grow so extensively and so quickly—“not even aggressive cancer cells,” says Jan Brosens, an obstetrician and gynecologist at the University of Warwick in the UK. As the tissue heals—in a rare example of scarless repair—it becomes an environment that can shield an embryo, which is a foreign entity in the body, from an immune system trained to reject interlopers.
Scientists have filled in the rough outline of this process after decades of research, but many details remain opaque. How exactly the endometrium repairs itself so extensively is unknown. Why some people have much heavier periods than others remains an open question. And why humans menstruate, rather than reabsorbing unused endometrial tissue like many other mammals, is a matter of hot debate among biologists.
This lack of understanding hampers scientists, who would like to find treatments for periods that are too painful to be tamed by over-the-counter painkillers or too heavy to be absorbed by pads and tampons. As a result, many people suffer. A study performed in the Netherlands found that on average women lost about a week of productivity per year because of abdominal pain and other symptoms related to their periods. “It would not be unusual for a patient to see me in the clinic and say that every month, they had to have two or three days off work,” says Hilary Critchley, a gynecologist and reproductive biologist at the University of Edinburgh.
Heavy periods can make even daily tasks difficult. Getting up from a chair, for example, can be an ordeal for someone worried about the possibility of having stained the seat. Mothers with low iron levels tend to have babies with low birth weights and other health problems, so the effects of heavy menstruation trickle down through generations. And yet the uterus often goes unacknowledged, even by researchers who are exploring topics like tissue regeneration, to which the organ is clearly relevant, Brosens says. “It is almost unforgivable, in my view,” he adds.
Ask researchers why menstruation remains so enigmatic and you’ll get a variety of answers. Most everyone agrees there’s not enough funding to attract the number of researchers the field deserves—as is often the case for health problems that primarily affect women. The fact that menstruation is shrouded in taboos doesn’t help. But some researchers say it has been hard to find the right tools to study the phenomenon.
Scientists tend to start studies of the human body in other organisms, such as mice, fruit flies, and yeast, before translating the knowledge back to humans. These so-called “model systems” reproduce quickly and can be altered genetically, and scientists can work with them without running into as many ethical or logistical concerns as they would if they experimented on people. But because menstruation is so rare in the animal kingdom, it’s been tough to find ways to study the process outside the human body. “I think that the main limitations are model systems, honestly,” says Julie Kim, a reproductive biologist at Northwestern University.
Early adventures
In the 1940s, the Dutch zoologist Cornelius Jan van der Horst was among the first scientists to work on an animal model for studying menstruation. Van der Horst was fascinated by unusual, poorly studied critters, and this fascination led him to South Africa, where he trapped and studied the elephant shrew. With a long snout reminiscent of an elephant’s trunk and a body similar to an opossum’s, the elephant shrew was already an oddball when van der Horst learned that it’s one of the few animals that get a period—a fact he probably discovered “more or less by accident,” says Anthony Carter, a developmental biologist at the University of Southern Denmark who wrote a review of van der Horst’s work.
Elephant shrews are not cooperative study subjects, however. They only menstruate at certain times of year, and they don’t do well in captivity. There’s also the challenge of catching them, which van der Horst and his colleagues attempted with hand-held nets. The shrews were agile, so it was “sometimes a fascinating but mostly a disappointing sport,” he wrote.
Around the same time, George W.D. Hamlett, a Harvard-based biologist, discovered an alternative. Hamlett was examining preserved samples of a nectar-loving bat called Glossophaga soricina when he noticed evidence of menstruation. The bats, which live primarily in Central and South America, were not easily accessible, so for several decades his discovery remained simply a point of interest in the scientific literature.
Then, in the 1960s, an eager graduate student named John J. Rasweiler IV enrolled at Cornell University. Rasweiler wanted to study a type of animal reproduction that mirrors what happens in humans, so his mentor pointed out Hamlett’s discovery. Perhaps Rasweiler would like to go find some bats and see what he could do with them?
With a long snout reminiscent of an elephant’s trunk and a body similar to an opossum’s, the elephant shrew was already an oddball when van der Horst learned that it’s one of the few animals that get a period.
“It was a very challenging undertaking,” Rasweiler says. “Essentially I had to invent everything from start to finish.” First there were the trips to Trinidad and Colombia to collect the bats. Then there was the issue of how to transport them back to the United States without their getting crushed or overheating. (Shipping them in takeout food containers, bundled together into a larger package, turned out to work well.) Once the bats were in the lab, he had to figure out how to work with them without letting them escape. He ended up constructing a walk-in cage on wheels that he could roll up to the bats’ enclosures.
“I loved working with them—delightful animals,” says Rasweiler, who has since retired from a career as a reproductive physiologist at SUNY Downstate. But other researchers were put off by the idea of working with a flying animal.
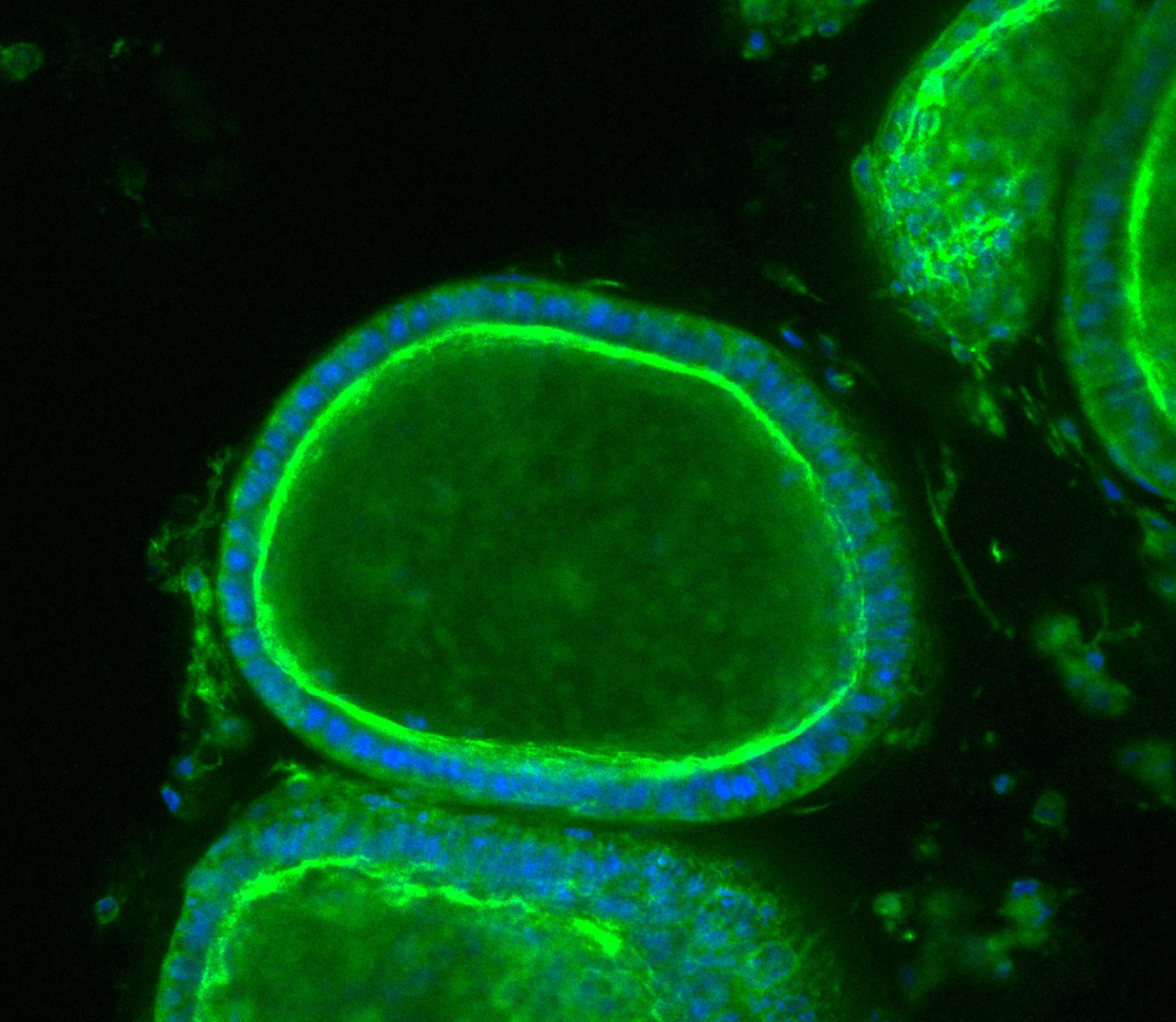
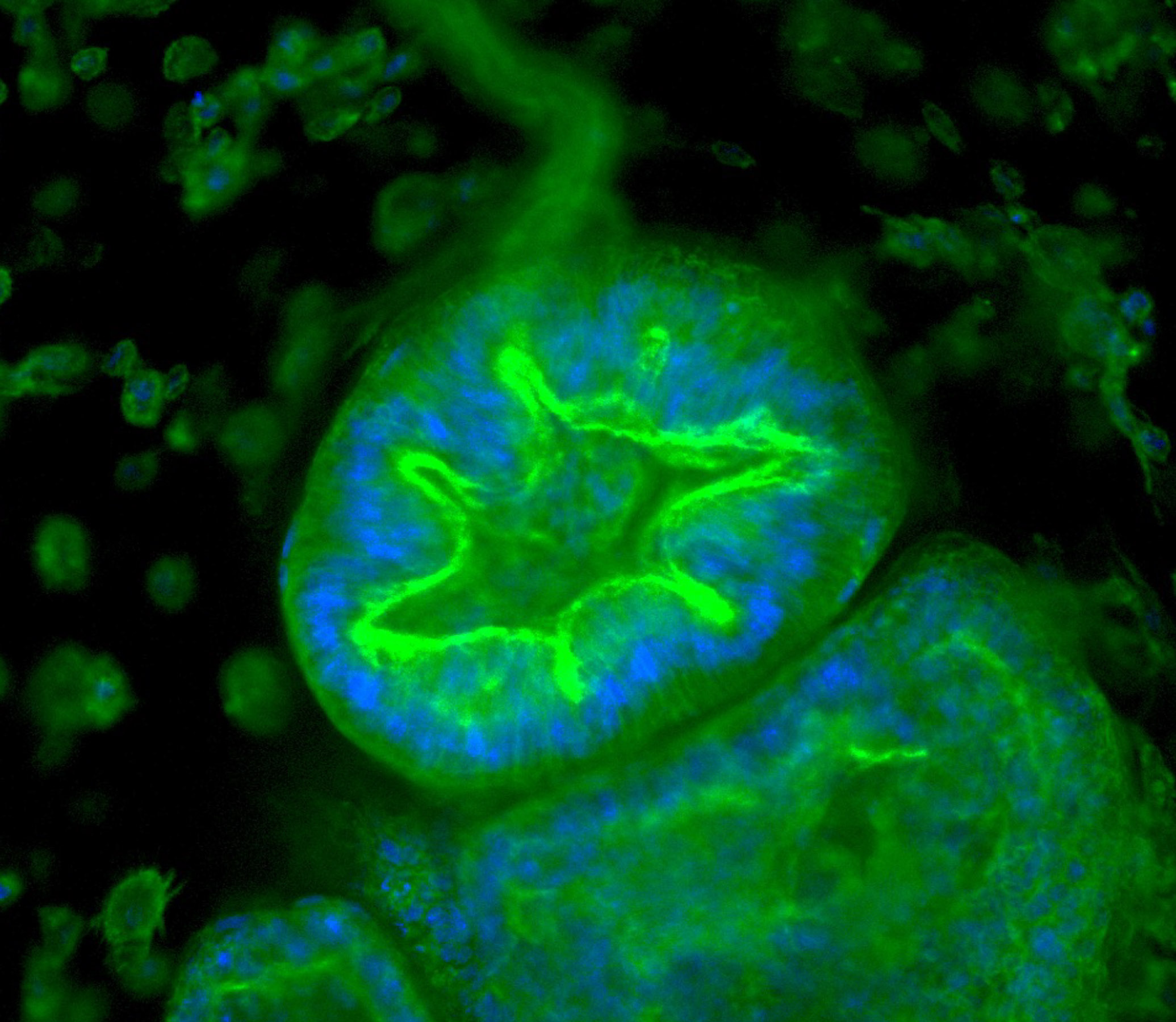
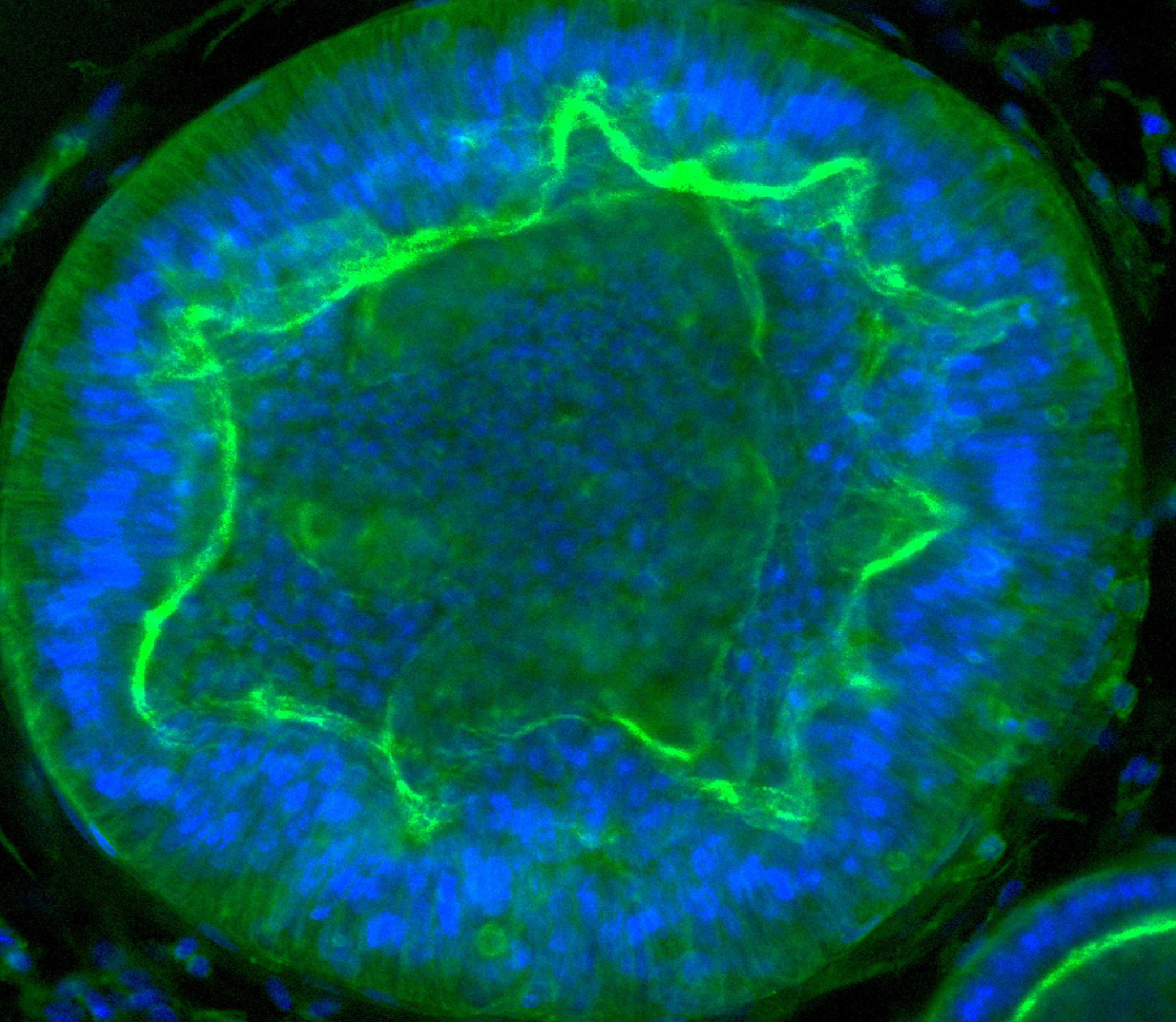
Researchers can track how organoids respond to various stimuli. Here endometrial tissue thickens when exposed to a synthetic version of the hormone progesterone, mirroring the lead-up to menstruation. Image source: “Organoid co-culture model of the cycling human endometrium in a fully-defined synthetic 2 extracellular matrix reveals epithelial-stromal crosstalk.” Juan S. Gnecco et al.
In 2016, the spiny mouse—a rodent that thrives in the dry conditions of the Middle East, South Asia, and parts of Africa—joined the exclusive club of animals known to menstruate. Spiny mice can be raised in the lab, so they may become valuable subjects for menstruation research. But millions of years of evolution lie between humans and mice, leading Brosens to think the genetics underlying their uteruses are likely to differ substantially.
Much of the foundational work on menstruation has been performed in macaque monkeys. But primates are expensive to care for, and the Animal Welfare Act places restrictions on primate research that do not apply to other common lab animals. Through a series of manipulations, scientists also found that they could force a common lab mouse to have something similar to a period. This model has been useful, but it’s still only an artificial representation of true human menstruation.
What researchers really needed was a way to use humans as study subjects for menstruation research. But even setting aside the obvious ethical concerns, such a thing would be very challenging logistically. The endometrium evolves exceedingly quickly—“at an hourly rate, we see different responses from the cells, different functions,” says Aleksandra Tsolova, a cell biologist at the University of Calgary. “It’s very dynamic tissue.” Researchers would need to perform invasive biopsies almost constantly to study it inside the human body, and even then, altering it genetically or through chemical treatments would be largely impossible.
But by the early 1900s, a solution to this problem had already started to emerge. And it was not a creature from the jungle or the African grasslands that paved the road, but an organism from the bottom of the sea.
Organoids come on the scene
The groundwork for what would become modern-day organoids was laid in 1910, when a zoologist named Henry Van Peters Wilson realized that cells from marine sponges have a sort of “memory” of how they’re arranged in the animal, even after they’re separated. When he dissociated a sponge by squeezing it through a mesh and then mixed the cells together again, the original sponge re-formed. Midcentury work showed that certain cells from chick embryos have a similar ability.
In 2009, a study published in the journal Naturedescribed a possible way of extending these observations to human organs. The researchers took a single adult stem cell from a mouse intestine—which had the ability to become any type of intestinal cell—and embedded it in a gelatinous substance. The cell divided and, together with its progeny, formed a miniature, simplified version of the intestinal lining. It was the first time scientists had laid out a method of creating an organoid from human tissue that was accessible to many labs and straightforward to adapt to other organs.
Since then, scientists have extended this general approach to mimic aspects of around a dozen human tissue types, including those from the gut, the kidneys, and the brain—and, by the late 2010s, the uterus.
It was a happy accident that brought endometrial organoids into the mix. In the years leading up to their development, scientists had been trying to study the endometrium by growing its cells in smooth layers on the bottoms of laboratory dishes. Stromal cells, which provide structural support for the tissue and play a key role in pregnancy, proved easy to grow this way—these cells secrete a substance that sticks them to each other, and also makes them adhere to petri dishes. But epithelial cells, another critical component of the endometrium, posed a problem. In a dish, they stopped responding to hormones, and their shapes were unlike what’s seen in the human body.
Then, while working with a mix of human placental and endometrial tissue in an effort to get the placenta to form organoids, a reproductive biologist named Margherita Turco noticed something serendipitous. If they were suspended in a gel instead of being grown in liquid, and given just the right mix of molecules from the human body, endometrial epithelial cells assembled into tiny three-dimensional simulacra of the organ they came from. “They grew really, really well,” Turco says. In fact, endometrial organoids were “kind of overtaking the cultures.” Another group independently published similar findings around the same time.
Today, placental and endometrial organoids are both valuable tools in the lab Turco runs at the Friedrich Miescher Institute for Biomedical Research in Basel, Switzerland. Her original 2017 publication calls for using tissue from a biopsy, rather than stem cells, to make organoids from the endometrium. Some labs instead use tissue removed from people who have had hysterectomies. But Turco’s lab recently showed that bits of the endometrium found in menstrual blood also work, which would mean the new endometrial organoids can be grown without requiring biopsies or surgery.
From all these starting points, researchers can now create microcosms of the human uterus. Each organoid reminds Tsolova of a tiny bubble suspended in a gelatinous dessert. And each presents a unique opportunity to understand processes that science has long ignored.
Period in a dish
Endometrial organoids became integral to the work of the small community of researchers focused on the uterus. Since 2017, many labs have put their own spins on these new tools.
Kim’s lab has added stromal cells to the epithelial cells that make up classic endometrial organoids. She and her colleagues mix the two together and simply let the combination “do its thing,” she says. The result is like a malt ball with stromal cells on the inside and epithelial cells on the outside.
In 2021, Brosens and his colleagues created similar structures, which they call “assembloids.” Instead of mixing the two cell types together, they created an organoid out of epithelial cells and then added a layer of stromal cells on top. Using assembloids, they’ve learned that deteriorating cells play a key role in helping the embryo implant in the uterus. Because the endometrium is constantly dying and regrowing, the tissue is highly flexible and able to adjust its shape, Brosens explains. This helps the tissue kick-start pregnancy: “Maternal cells will grab the embryo,” he says, “and literally pull that embryo into the tissue.”
A video from one of Brosens’s recent publications shows an assembloid remodeling around a five-day-old embryo. Before he and his colleagues did this work, conventional wisdom said the endometrium was passive tissue that was invaded by the embryo, but that’s “just completely wrong,” he says. This new understanding of how embryos implant could improve in vitro fertilization and help explain why some people are prone to miscarriages.
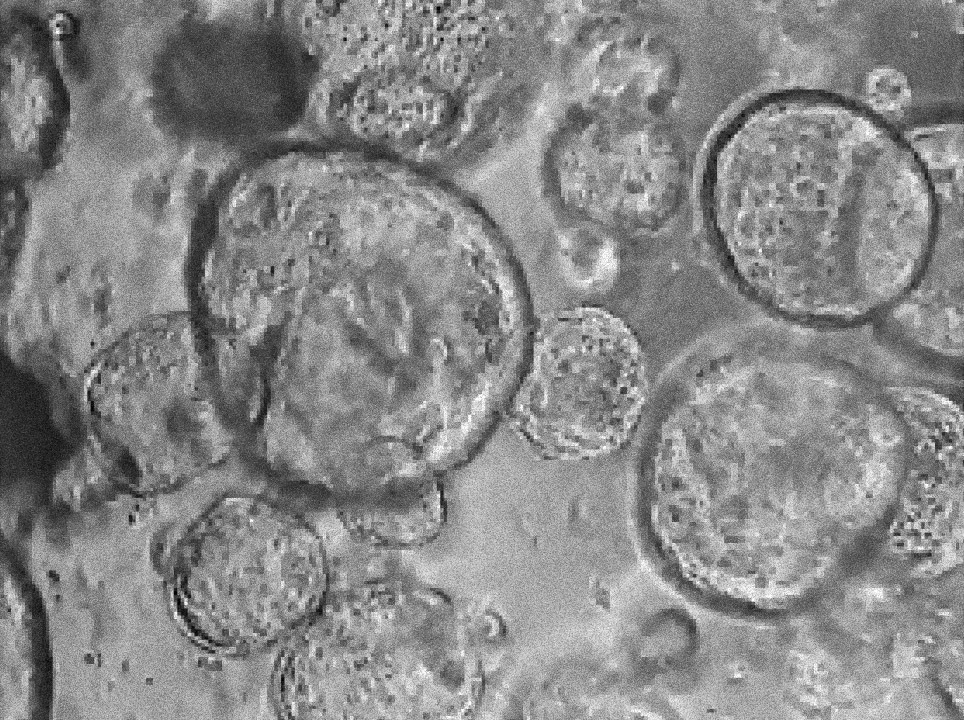
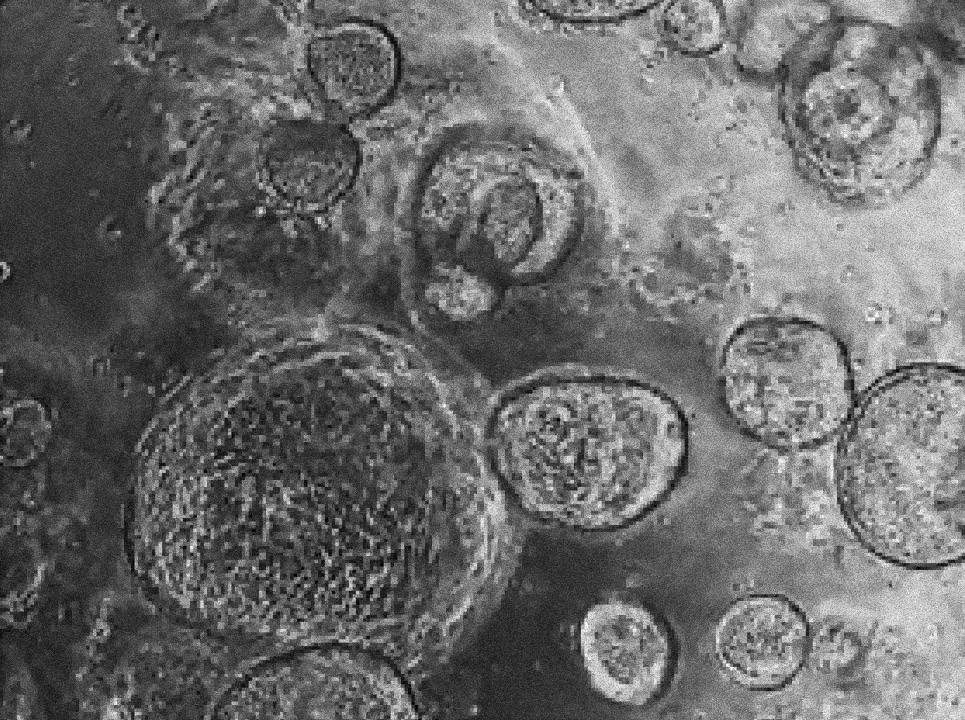
Margherita Turco's laboratory at the Friedrich Miescher Institute for Biomedical Research in Switzerland has found that organoids derived directly from the endometrium (first image) and from menstrual blood (second image) of the same person have indistinguishable shapes and structures. Image source: “Menstrual flow as a non-invasive source of endometrial organoids.” Tereza Cindrova-Davies et al. communications biology.
Eventually, Critchley hopes, scientists can design treatments that let people choose when to have a period—or if they even want to have one at all. Hormonal birth control can accomplish these goals for some, but these drugs can also cause unscheduled bleeding that makes periods harder to manage, and some people find the side effects of the medication intolerable.
To create better options, scientists still need to understand how a normal period works. Making an organoid menstruate in a dish would be a huge boon for achieving this goal, so that’s what some researchers are trying to do.
By manually adding hormones to organoids, Gnecco and his collaborators can replicate some of what the endometrium experiences over the course of a month. As the cycle progresses, they see the cells adjusting the complement of genes they use, just as they would in the human body. The shape of the organoid also follows a familiar pattern. Glands—infoldings of cells from which mucus and other substances are secreted—change from smooth tubes to sawtooth-like structures as this faux menstrual cycle progresses.
“It’s mind-blowing that we are very, very close to the patient, but we’re not working within the patient. There’s huge potential.”
Aleksandra Tsolova, cell biologist, University of Calgary
With this system working, the next step is to figure out what happens when the endometrium malfunctions. “That’s what really got me excited,” Gnecco says. As a first step, he treated organoids with an inflammatory molecule called IL-1β, which is a hallmark of the lesions that characterize endometriosis. IL-1β caused organoids to grow rapidly, but only when stromal cells were mixed in along with the epithelial cells. This suggests that signals from stromal cells might be part of what causes endometriosis to develop into a painful condition.
Meanwhile, Kilinc is trying to understand why some people’s periods are so heavy. Endometrial tissue growing into the muscle that lines the uterus seems to cause lesions, which can be one source of excessive bleeding. To see how such lesions could form, Kilinc watches how endometrial organoids react when they hit a dense gel, which mimics the texture of muscle.
In a soft gel, endometrial organoids maintain a nice, round structure. But when the organoid is in a stiff gel, it’s a different story. A video from one of Kilinc’s recent experiments shows an organoid pulsating and squirming, almost like a pot of water that’s about to boil over. Finally, a group of cells shoots off, creating an appendage-like structure that punctures the stiff gel. Videos like this make Kilinc think that contact with muscle might be among the triggers that cause the endometrium to start wounding this tissue and causing heavy bleeding. “But,” she adds, “this is not clear yet—we are still investigating.”
Speedier science
Today’s endometrial organoids can’t do everything animal models can do. For one thing, they don’t yet include key components of menstruation, like blood vessels and immune cells. For another, they can’t reveal how distant parts of the body, like the brain, influence what happens in the uterus. But because they’re derived from human tissue, they’re intimately related to the bizarre, idiosyncratic process that is a human period, and that’s worth a lot. “It’s mind-blowing that we are very, very close to the patient, but we’re not working within the patient,” Tsolova says. “There’s huge potential.”
In parallel to the work on organoids, scientists have created an “organ on a chip” that mimics the endometrium. Tiny tubes affixed to a flat surface carry liquids to endometrial tissue, mimicking the flow of blood or hormones transmitted from other parts of the body. An ideal model system could combine endometrial cells in their natural arrangement—as in an organoid—with flowing liquids, as on a chip.
Already, organoids have helped researchers solve old puzzles. Researchers in Vienna, for example, used this technology to figure out which genes cause some endometrial cells to grow cilia—hair-like structures that beat in coordination to move liquid, mucus, and embryos within the uterus. Other researchers have used organoids to learn how endometrial cells mature throughout the menstrual cycle. Meanwhile, Kim and her colleagues used organoids to study how the endometrium responds to abnormal hormone levels, which may be a factor in endometrial cancer.
People who menstruate have waited a long time for researchers to tackle such questions. Burdensome periods are often seen as just a “women’s problem”—a mindset Tsolova disagrees with because it ignores the fact that people struggling with menstruation often can’t contribute their full range of talents to their communities. “It’s a societal problem,” she says. “It affects every person, in every way.”
Saima Sidik is a freelance science journalist based in Somerville, Massachusetts.
Deep Dive
Biotechnology and health
How scientists traced a mysterious covid case back to six toilets
When wastewater surveillance turns into a hunt for a single infected individual, the ethics get tricky.
An AI-driven “factory of drugs” claims to have hit a big milestone
Insilico is part of a wave of companies betting on AI as the "next amazing revolution" in biology
The quest to legitimize longevity medicine
Longevity clinics offer a mix of services that largely cater to the wealthy. Now there’s a push to establish their work as a credible medical field.
There is a new most expensive drug in the world. Price tag: $4.25 million
But will the latest gene therapy suffer the curse of the costliest drug?
Stay connected
Get the latest updates from
MIT Technology Review
Discover special offers, top stories, upcoming events, and more.