Novavax has announced encouraging early results for its experimental coronavirus vaccine
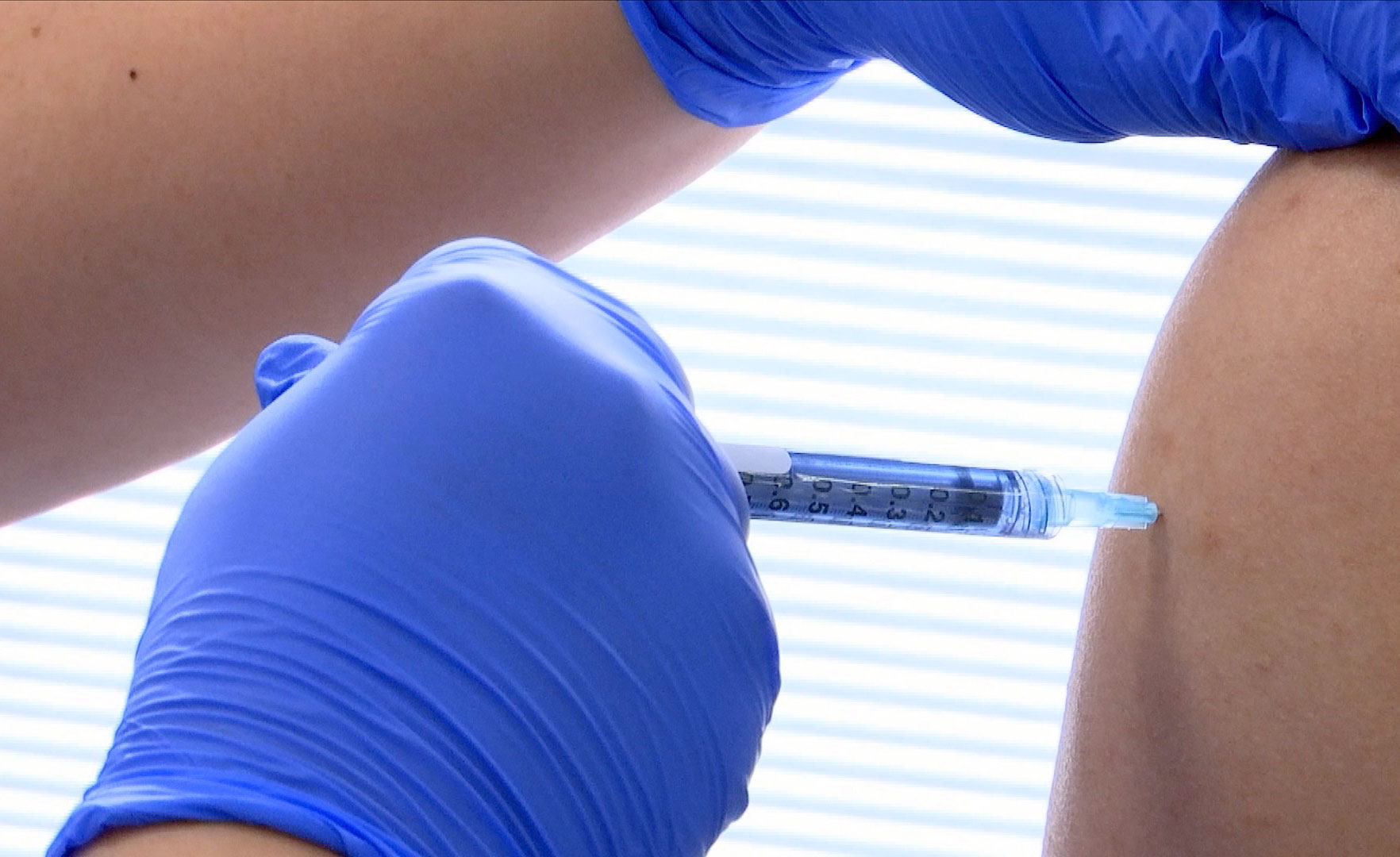
The news: Maryland biotechnology company Novavax has announced encouraging results from a preliminary study of its experimental coronavirus vaccine. The trial enrolled 131 healthy volunteers in Australia, gave them either a placebo or one of four escalating doses of its vaccine, and found that everyone who received the vaccine produced a high level of antibodies against covid-19. Novavax signed a deal last month to receive $1.6 billion in funding as part of the US federal government’s “Operation Warp Speed” program to develop a covid-19 vaccine.
Any side effects? About 80% volunteers experienced pain and tenderness at the injection site, while more than 60% had other side effects like fatigue and headaches. Most reactions were mild, but eight patients had severe side effects. Novavax says no one was hospitalized as a result, and all of the reactions disappeared after a few days.
Some provisos: As with any Phase I trial, the aim was to establish the safety rather than the efficacy of the vaccine. The data has been published on a preprint server, which means it hasn’t been peer-reviewed yet. Much like vaccine study results recently published by the Oxford/AstraZeneca team, and the Moderna trial, it’s too early to draw firm conclusions about whether the vaccine will protect against coronavirus long-term. That’s why Moderna and AstraZeneca are now enrolling tens of thousands of volunteers for the next stage of trials, when we’ll get a clearer picture of just how likely these vaccines are to work.
The bigger picture: AstraZeneca’s biopharma lead, Mene Pangalos, has warned that, rather than conferring permanent protection, a covid-19 vaccine may only have an effect for a year or two. There are more than 150 vaccines being developed around the world, with at least 25 already in human trials. Given the virus has infected 18.5 million people globally and killed over 700,000, the pressure is on to find a viable solution—although scientists warn that clinical safety cannot be compromised in the face of political and nationalistic pressure.
Deep Dive
Biotechnology and health
How scientists traced a mysterious covid case back to six toilets
When wastewater surveillance turns into a hunt for a single infected individual, the ethics get tricky.
An AI-driven “factory of drugs” claims to have hit a big milestone
Insilico is part of a wave of companies betting on AI as the "next amazing revolution" in biology
The quest to legitimize longevity medicine
Longevity clinics offer a mix of services that largely cater to the wealthy. Now there’s a push to establish their work as a credible medical field.
There is a new most expensive drug in the world. Price tag: $4.25 million
But will the latest gene therapy suffer the curse of the costliest drug?
Stay connected
Get the latest updates from
MIT Technology Review
Discover special offers, top stories, upcoming events, and more.