Forget Buckyballs, Here Comes Volleyballene
Buckyballs are all the rage these days given their stability and unique chemical properties. The classic football-shaped molecule consists of 60 carbon atoms arranged in 20 hexagons and 12 pentagons, but chemists have observed various other configurations. C72, C76, and C84 are fairly common, and some buckyballs have up to 100 carbon atoms.
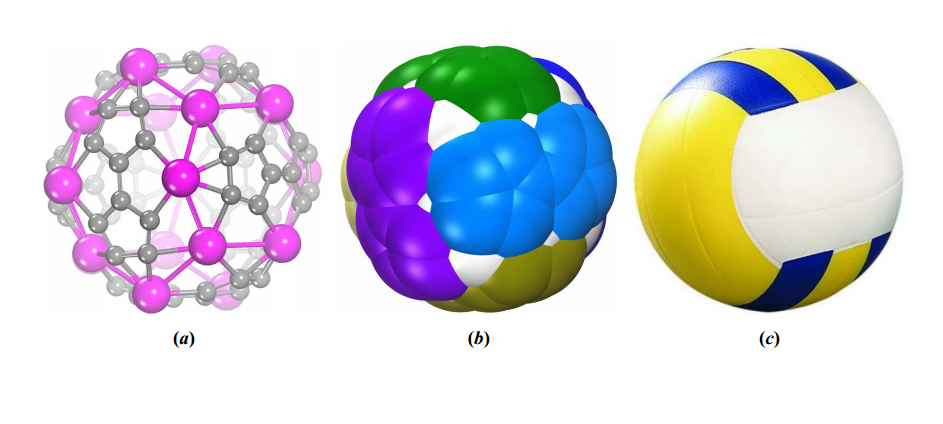
Recently, chemists have begun to replace some of the carbons with other atoms such as titanium and vanadium, creating buckyball variants. And today, Jing Wang at Hebei Normal University in China and a few pals predict an even more exotic version.
They’ve used molecular simulations to study the properties of a fullerene consisting of 60 carbons and 20 scandium atoms. What makes this molecule volleyball-like is that it is composed of six subunits that are essentially stitched together in a criss-cross pattern to form a ball (see image above). That’s just the same way that volleyballs are stitched together (in principle, at least). Each subunit is made up of eight scandium atoms and 10 carbons.
The simulation gives a remarkably detailed picture of the properties of the new molecule. Jing and co have simulated the character of the bonds that hold it together, their binding energy, their vibrational frequencies, and the stability of the structure.
And they say volleballene is clearly the most stable of all the structures that Sc20C60 can form. The tam’s vibrational analysis suggests that volleyballene should be stable when heated and remarkably stable chemically too.
In other words, volleyballene is a molecule waiting to be synthesized. So if you’re a chemist with a little time on your hands, let us know when you’ve made one of these things.
Ref: arxiv.org/abs/1502.03507 : Sc20C60: A Volleyballene
Keep Reading
Most Popular
Large language models can do jaw-dropping things. But nobody knows exactly why.
And that's a problem. Figuring it out is one of the biggest scientific puzzles of our time and a crucial step towards controlling more powerful future models.
How scientists traced a mysterious covid case back to six toilets
When wastewater surveillance turns into a hunt for a single infected individual, the ethics get tricky.
The problem with plug-in hybrids? Their drivers.
Plug-in hybrids are often sold as a transition to EVs, but new data from Europe shows we’re still underestimating the emissions they produce.
Stay connected
Get the latest updates from
MIT Technology Review
Discover special offers, top stories, upcoming events, and more.