Buckyballs are all the rage these days given their stability and unique chemical properties. The classic football-shaped molecule consists of 60 carbon atoms arranged in 20 hexagons and 12 pentagons, but chemists have observed various other configurations. C72, C76, and C84 are fairly common, and some buckyballs have up to 100 carbon atoms.
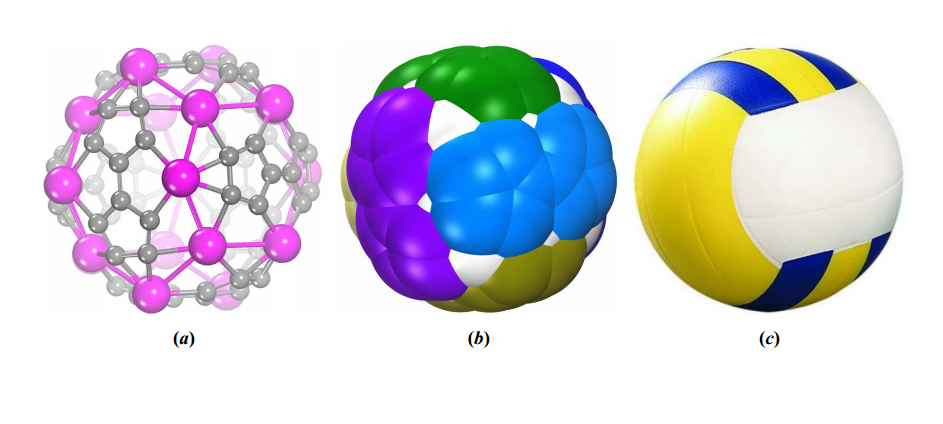
Recently, chemists have begun to replace some of the carbons with other atoms such as titanium and vanadium, creating buckyball variants. And today, Jing Wang at Hebei Normal University in China and a few pals predict an even more exotic version.
They’ve used molecular simulations to study the properties of a fullerene consisting of 60 carbons and 20 scandium atoms. What makes this molecule volleyball-like is that it is composed of six subunits that are essentially stitched together in a criss-cross pattern to form a ball (see image above). That’s just the same way that volleyballs are stitched together (in principle, at least). Each subunit is made up of eight scandium atoms and 10 carbons.
Don’t settle for half the story.
Get paywall-free access to technology news for the here and now.