I tried Prolon’s starvation diet so you wouldn’t have to
A diet based on caloric restriction might make you live longer. It’ll certainly feel like longer.

My bitterness peaked midway through day four of the “Fast-Mimicking Diet,” when a parent arrived at my daughter’s softball game with doughnuts. As little girls and fellow coaches crowded around the box, I stood apart, glumly sipping out of my special water bottle with its “proprietary” blend of nutrients.
For breakfast, I’d consumed a nut bar the size of a small cracker and a couple of vitamins. Lunch was five olives from Seville.
Frankly, I’d begun to resent Valter Longo, the inventor of Prolon, the five-day, $250 fad diet causing my misery. True, the Italian-born biochemist had seemed perfectly nice when I’d reached him at his office at the University of Southern California’s Longevity Institute a few days before to speak with him about the science behind the diet and what it might do for my general health and longevity. He had patiently explained how the diet would temporarily shift my body into a starvation state that would prompt my cells to consume years of accumulated cellular garbage before unleashing a surge of restorative regeneration. Getting rid of garbage had sounded like just what I needed. But now I blamed him for my predicament. I wanted a doughnut.
My Prolon “meal kit” had arrived in a white cardboard container a little bigger than a shoebox. Inside I’d found a meal program card spelling out the menu, a large empty water bottle emblazoned with the word “Prolon,” and five smaller cardboard boxes, each labeled with a corresponding day. I opened the box for day one, billed as a higher-calorie “transition day,” and was pleasantly surprised. It didn’t look so bad. I’d be sampling many of the diet’s highlights: a small packet of kale crackers, powdered tomato soup blend, algae oil supplements, a bag of olives, herbal tea, and not one but two nut-based bars (albeit distressingly small).
When I opened up day two, however, I began to get a better sense of what I was in for. One of the puny nut bars had been replaced by a glycerin-based “energy” drink, which I was instructed to add water to and sip on throughout the day. There was more herbal tea—hibiscus, mint, and lemon (I don’t even like herbal tea)—plus a couple more powdered-soup packs and two tiny packets of olives. Where was the rest of it?
“We give you quite a bit of food—just over 800 calories,” a trim, youthful nutritionist explained unironically in a YouTube video I pulled up to make sure there was no mistake. The goal of Prolon, he explained, is to trick the body into thinking you are fasting, prompting it to “suppress all of the same pathways as if you were doing a full fast.”
“By day three your body has activated all of the benefits and then spends the rest of the days optimizing, regenerating, rejuvenating,” he added cheerfully. “So you can really expect to feel the benefits on day four.”

The lesson of Biosphere 2
The idea that starving yourself while still taking in crucial nutrients will let you live longer is not new. The practice, called caloric restriction, is the only proven way to extend life that works in a wide variety of creatures, from worms to rodents to primates. And it was already of interest to biologists when Longo was first starting out in the field, almost 30 years ago.
At the time, there were few people more identified with the radical diet than Roy Walford. A larger-than-life figure, Walford had already demonstrated in his UCLA lab that he could double the life span of mice by drastically restricting their caloric intake. He had also published a number of popular books on the topic, among them The 120 Year Diet and Beyond the 120 Year Diet, and would follow a strict 1,600-calorie diet himself for the last 30 years of his life (the US Department of Health recommends 2,800 calories a day for an active middle-aged man). He weighed in at 130 pounds (59 kilograms) for most of that time, far below the average weight for someone 5'9" (175 centimeters) tall.
When Longo arrived in Walford’s lab to begin his PhD work in 1992, Walford was on temporary leave. Several months earlier, he’d gone to the Arizona desert to serve as one of eight “crew members” in a three-acre (1.2-hectare) complex of hermetically sealed domes known as Biosphere 2. The two-year experiment in communal living was billed as a test of the kind of home that might one day be built and used for the colonization of space. Soon after entering the biosphere in 1991, the crew members discovered they could not grow nearly as much food as they had anticipated. It was Walford, the crew physician, who persuaded them to follow a severe calorie restriction diet—a decision that garnered worldwide media attention as they staggered out of the biosphere in 1993, gaunt and sickly.
Walford died in 2004, at 79, of amyotrophic lateral sclerosis, a.k.a. motor neuron disease or Lou Gehrig’s disease—a condition, Longo notes, that many suspected was the result of the two years of extreme calorie restriction he endured in the biosphere. It’s a theory Longo takes seriously.
“We don’t know if that was the cause,” he says. “But I was there when he came out of the biosphere, and he looked sick and so did everybody else. Maybe he paid a price for that. We don’t know what the connection is with motor neuron disease. But it’s possible his neurons couldn’t handle this extreme situation for years and years and years. Maybe it combined with something else.”
The lesson was clear: while caloric restriction might make you live longer, doing it for extended periods was a problem, and probably not practical for most people.
Biological house cleaning
In any case, at the time Longo was less interested in the association between diet and longevity than in what he considered to be a fascinating by-product of extreme calorie restriction. Longo discovered that when he starved bacteria and yeast, they not only lived far longer than their well-fed counterparts but entered a protective state that seemed to shield them from environmental stress. When exposed to hydrogen peroxide, yeast in starvation mode were between 60 and 100 times more resistant to cellular damage than yeast that had been taken from an environment rich in glucose to feed on.
That was surprising. Wouldn’t a cell weakened by starvation become less resistant to damage, rather than more? But in the years that followed, a consensus emerged that explained both Longo’s discovery and other researchers’ findings that lab animals fed a calorie-restricted diet lived longer.
“We give you quite a bit of food—just over 800 calories,” a trim, youthful nutritionist explained unironically in a helpful YouTube video I pulled up to make sure there was no mistake.
In a well-fed state, our cells and those of other multicellular organisms invest energy in reproduction and regeneration. But when food is scarce, those functions shut down, and the cell diverts its energy to feeding and protecting itself; it takes far less energy to protect the cells you already have than to build new ones.
To do so, the body revs up a host of protective pathways. In the case of Longo’s yeast and bacteria (and eventually mice), he and others would later show, the organisms make enzymes that neutralize free radicals—molecules with unpaired electrons that can damage other cells. Other proteins and enzymes are produced that ensure proteins don’t misfold, and in every cell, the cellular machinery devoted to repairing its own DNA kicks into overdrive.
In more complex organisms like mice or humans, the body still needs calories to keep the heart beating, the brain thinking, and the muscles contracting. To get them, it engages in a process called autophagy (an ancient Greek word that means “self-consumption”), breaking down the body’s own cells and recycling their components. But this autophagy is not random.
“It tends to begin by eating proteins that are misfolded or denatured,” explains Eric Verdun, the president and CEO of the Buck Institute for Research on Aging. “There is a house-cleaning aspect to it. It consumes itself, but it consumes the proteins that need to be cleaned out first.”
Forced to turn inward for energy sources, the body hunts down, eats, and recycles its own cellular garbage, in the process removing debris that can prevent cells from operating efficiently.
Starving cancer cells
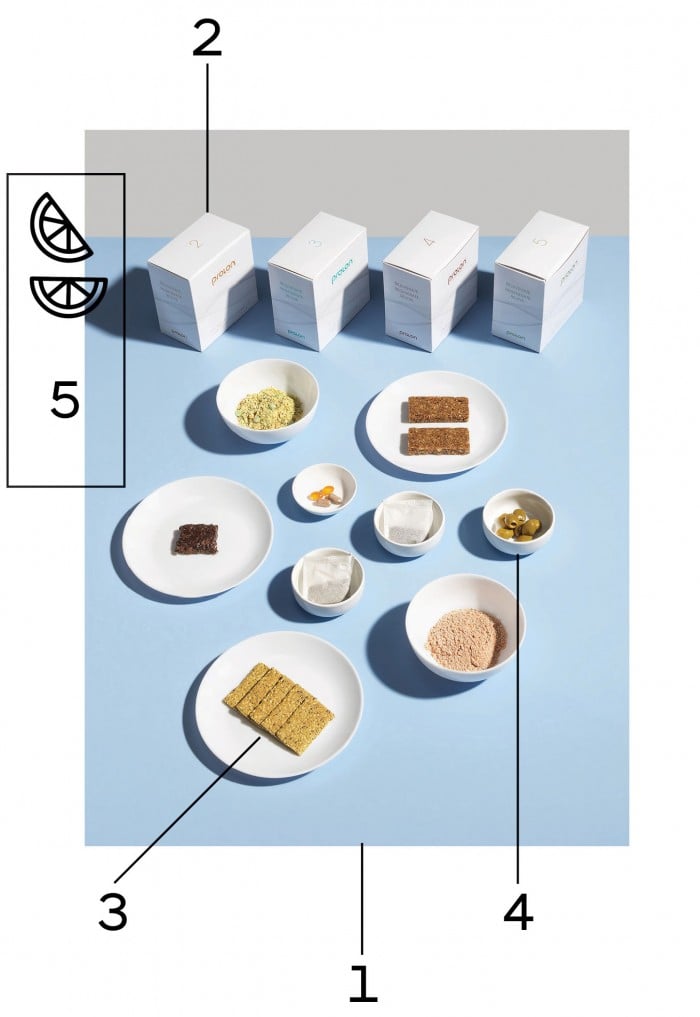
1. The diet tricks your body into starvation mode, but tries to make it tolerable.
2. Each day of food comes in its own distressingly small cardboard box.
3. Kale crackers were the author’s favorite.
4. Prolon is big on olives from Seville, which taste great but are not very filling.
5. You are allowed to add lemon to enhance the flavor if necessary.
Longo was fascinated by this process, and he would spend the next two decades helping to identify the genes and biological pathways at work. As he did so, he began to recognize something unexpected. Many of the genes involved were also prominent in the cancer literature.
In the cancer field, they were known as “proto-oncogenes”—the very same genes that, when mutated, had the power to transform a normal cell into a cancerous one, by essentially wedging the cell’s regeneration machinery permanently into the “on” position and causing it to divide and proliferate uncontrollably.
That gave Longo an idea. He had already shown that starvation could cause all an organism’s normal cells to enter a protective state. But cancer cells aren’t normal cells. One of the hallmarks of cancer is that the cells do not respond to biochemical signals suppressing their growth. What would happen, Longo wondered, if he put mice into starvation mode before exposing them to chemotherapy? If the normal cells went into a protective state but the cancerous ones did not, drugs could kill the cancer with less risk of damaging normal tissue.
Longo administered high doses of the chemotherapy drug doxorubicin to yeast. He found that under starvation conditions, normal yeast cells became a thousand times more resistant to stress, while cancer cells were exposed to the full brunt of the poisons. When Longo repeated the test on mice, starving one group for 60 hours prior to the chemo, the results were dramatic. Every single one of the normal mice died. Every single one of the starved mice lived.
But when Longo began reaching out to clinicians who worked with cancer patients, he encountered unexpected resistance. “We thought, ‘Of course. Everybody is going to do it. It’s going to be easy,’” Longo recalls. “It took us five years to recruit 18 patients. It was water-only fasting. Completely free. Don’t eat. Just drink. Nobody wanted to do it. Everybody thought it was a disaster.”
Facing defeat, Longo and his team groped for alternatives and quickly hit on a better idea: perhaps they could design a diet that aimed to trick the body into thinking it was fasting, without actually starving. Longo knew that if he made a low-carbohydrate diet lacking glucose and certain key amino acids—in other words, most proteins and all carbs were out—the body would still enter its protective state.
Longo created a company, L-Nutra. By 2014, his lab had produced its first prototype. And in 2015, he published a study demonstrating that middle-aged mice on the fast-mimicking diet had far fewer tumors and were protected against cognitive decline. By then researchers in Leiden in the Netherlands had finally signed up enough volunteers to show that water-only fasting helped protect human patients from the ravages of chemotherapy. They agreed to begin testing a version of Longo’s diet on 125 cancer patients undergoing a similar chemo regime.
Longo says more than 40 trials are currently under way, at a wide variety of institutions. Not all of them are for cancer; there are also studies for Crohn’s disease, Alzheimer’s, and Parkinson’s.
The danger of success
Longo never forgot his roots in Walford’s lab. He knew that calorie restriction had “incredible effects,” but he also knew a strict diet had problems. Immunity was lowered, because the body could not produce white blood cells as quickly. Besides, “very few people can stick to calorie restriction,” he says. “Maybe one in ten thousand in the United States. It was not feasible for the great majority of the people.”
Longo was convinced, however, that periodic calorie restriction had some of the same health benefits as being on the diet full time—benefits worth the effort, if one could endure hunger pangs for a few days.
He decided he had to commercialize the diet—not just for the benefit of cancer patients, but because he also wanted it taken seriously as a tool for promoting healthy aging. “To me, it was very clear that it had to be somehow made into a drug-like product,” he says. “I realized early on that if there wasn’t a product, it would be very difficult for doctors and every health-care professional to take it seriously, and also to implement it. Doctors are used to something that has been tested clinically. They can’t say, ‘Here is a diet somebody at USC used.’”
So in September 2016, Prolon, the diet I tried, was born. Research on the “fast-mimicking diet” is still limited. So far, the most prominent publication on it is a 2017 study in the journal Science Translational Medicine, in which 71 healthy adults in the United States were given the Prolon diet for five consecutive days once a month for three months. The results established that the diet not only was safe but reduced things like body fat, blood pressure, insulin-like growth factor, low-density-lipoprotein cholesterol, and triglycerides, all of which are associated with aging and age-related diseases. It’s also far easier to stick to than a straight water starvation fast.
The sparseness of research data in humans has done little to dampen enthusiasm. Today Prolon is successful beyond anything an academic could reasonably hope for. The product—which, according to a company website, promises to “[put your body] into a protective and stress resistant mode; remove damaged cells and tissues; and promote self-repair through cellular regeneration and rejuvenation”—is all the rage in Silicon Valley. It’s sold in 15 countries and has been tried by more than 150,000 people.
Instead of doing backflips, however, Longo has grown increasingly concerned in recent years about what this commercial success might do to his scientific reputation. In 2017, after a series of articles about the product—one of which described Longo as “sounding like a snake-oil salesman” although it was fairly positive about the research—he announced he would no longer accept consulting fees and would donate his shares in the company to charity.
“All the decisions are made by the CEO,” he says. “I act as a professor … I am a scientist, and my heart is in the science and making sure it works. And the company’s heart is in a different place. Once you start having investors and you start having shareholders, it’s different.” He adds, “If I’m doing anything, I’m trying to get the company to do the right things, and sometimes I’m telling them, ‘Look, can you lower the price?’ I’m fighting for the people I see coming to the university to do the trials. I’m the watchdog of the company.”
Longo is not the only anti-aging scientist who has found himself the subject of unflattering media coverage, or attacks from rivals criticizing products he’s involved with as untested. The others deal with it in different ways. Nir Barzilai, who directs the Institute for Aging Research at the Albert Einstein College of Medicine, founded a publicly traded company called CohBar that focused on peptides involved in aging and age-related diseases. He stopped doing any research in the area to eliminate the appearance of conflict when he talks about it in the media. He has a financial stake, in other words, but his scientific career is now focused on other questions.
“You get into lots of conflict,” Barzilai says. “I’m in interviews. I’m on television. I didn’t want anybody to say, ‘You’re promoting your research and your company.’”
Others take a more relaxed attitude. Leonard Guarente, an MIT professor and prominent anti-aging researcher, cofounded a company called Elysium to sell supplements designed to work on a family of proteins called sirtuins that have a role in aging, as he discovered in the early 2000s. His stated goal is to use the profits to follow up with scientific studies that document the effects on humans. He’s not afraid to own it, despite the media backlash. “I don’t know if it bothers me as much as it bothers some others,” he says.
In a field badly tarnished by hype and false claims, the scientists face a real dilemma. Their products, unlike many others on the market, have legitimate science behind them. It’s early days, but their anti-aging approaches could work. “Our goal in this research is to stop the age-related diseases,” says Barzilai. “If we’re not going to do that, who’s going to do it, exactly? It cannot happen without us.”
Minus 8 pounds and happy
After five long days on Prolon, I awoke one morning to a day that promised as much soup, juice, and light meals of legumes and pasta as I could handle. Day six is a “transition” day, and dieters are encouraged not to binge. I can’t say I followed the suggested instructions. My first stop was Whole Foods, where I consumed an entire packet of Frisbee-size puffed-rice discs.
I felt great. My wife told me I seemed to be unusually energetic. I had lost eight pounds (nearly four kilograms) over five days, too. Overall, it hadn’t been too bad. I’d been reading about and reporting on different biological pathways involved in healthy aging on and off for several years, and the scientific claims made about Prolon were consistent with much of what I had read.
It wasn’t easy. I’d been hungry, grouchy, and bitter. But I never could have completed a real water-only fast for five days. And in the days that followed, it seemed to me I really did feel far better than I had before. Even if I was imagining the effects, which I don’t think I was, I stayed away from sugars and junk food for weeks afterward. That alone is reason enough to do it again—which I plan to after the suggested three-month interval has passed.
By then, the softball season will be long over.
Adam Piore is a freelance journalist based in New York. He is the author of The Body Builders: Inside the Science of the Engineered Human, about how bioengineering is changing modern medicine.
Deep Dive
Biotechnology and health
How scientists traced a mysterious covid case back to six toilets
When wastewater surveillance turns into a hunt for a single infected individual, the ethics get tricky.
An AI-driven “factory of drugs” claims to have hit a big milestone
Insilico is part of a wave of companies betting on AI as the "next amazing revolution" in biology
The quest to legitimize longevity medicine
Longevity clinics offer a mix of services that largely cater to the wealthy. Now there’s a push to establish their work as a credible medical field.
There is a new most expensive drug in the world. Price tag: $4.25 million
But will the latest gene therapy suffer the curse of the costliest drug?
Stay connected
Get the latest updates from
MIT Technology Review
Discover special offers, top stories, upcoming events, and more.