Organoids Proposed to Screen Patients for High-Priced Drugs
In May, the Dutch health minister, Edith Schippers, broke off negotiations with the biotech company Vertex. The problem was the eye-watering price of its blockbuster cystic fibrosis drug, Orkambi.
Too expensive, the ministry said. And it doesn’t work well enough. Patients would have to struggle on without it.
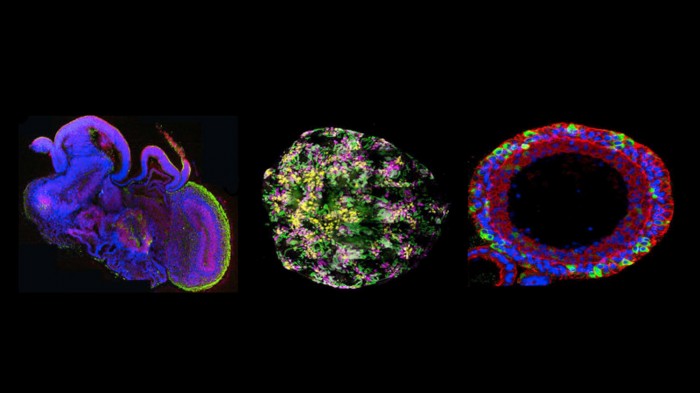
But now a coalition of patient groups, insurers, and Dutch biologists think a new technology could break the economic deadlock. They hope to make the drug more cost-effective by testing it in organoids, tiny clumps of cells that roughly resemble the components of human organs (see “10 Breakthrough Technologies: Brain Organoids”). How small is an organoid? Not much larger than the period in this sentence.
Last week, Dutch scientists approached the ministry with a proposal to grow such mini-organs in their laboratories, using cells obtained from all 1,500 Dutch cystic fibrosis patients. That way, they say, Orkambi and other costly drugs can be tested in the lab to see if they’ll be effective in a particular patient. Eventually, the drug would be paid for only if a patient's organoid responds.
“The organoid acts like an avatar for the patient,” says Hans Clevers, a scientist at the Hubrecht Institute who’s leading the effort. “You can expose the mini-organ to these very expensive drugs. Then you’d only give the drug to people who would really benefit.”
The plan, if it moves forward, could mark the start of a significant transition in the drug business. For the first time, blob-like organoids, not just blood tests or symptoms, might be widely used to make decisions about who gets—and who doesn’t get—the world’s most expensive new drugs.
Orkambi is designed for cystic fibrosis patients whose illness is caused by a specific type of gene mutation. Vertex says its largest studies of Orkambi show that everyone with that mutation responds. That means the company isn’t buying into the organoid payment plan just yet: it says large studies in volunteers are still the “gold standard” and “should not be superseded” by organoid results. “We’d be cautious that the science of organoids is still early," says Mark Higgins, senior medical director for cystic fibrosis at Vertex, which is based in Boston.
The Dutch project originated in Clevers’s lab and at the University Medical Center in Utrecht, where scientists mastered how to take a sample from a patient’s rectum and grow tiny bits of bowel tissue, complete with folded crypts and finger-like villi similar to those found in a person’s lower intestine.
The new testing approach involves exposing organoids to cystic fibrosis drugs and seeing if they swell up. If they do, it means the problem at the root of the disease is being reversed. Right now, however, no one knows how well organoids predict a patient’s long-term response. “It’s definitely not clear how accurate they are,” says Finn Hawkins, a professor at Boston University who works on lung organoids. “But I’ll be honest with you, I think it’s the future of medicine.”
In Holland, a nonprofit called the HUB Foundation for Organoid Technology is commercializing Clevers’s technology. Its customers include Vertex, which is already exploring organoids, at least in its research. The company now has two clinical trials running, one in Holland and one starting in Israel, in which the Dutch team is building organoids for patients. The aim is to figure out the connection between how organoids respond and which patients eventually benefit, and by how much.
Cystic fibrosis may be the perfect disease to try out the technology. The ailment, which produces sticky buildup in the lungs and leads to premature death, is caused by errors in a gene with a role in cells that make mucus, tears, and saliva. But more than 2,000 different mutations can cause cystic fibrosis, and drug companies can’t work on all of them.
Instead, drug makers have been racing to create precision drugs for the most common mutations. Vertex has two such drugs. One, Kalydeco, can help about one in 25 patients. Orkambi is the other: it’s approved for the most common mutation causing cystic fibrosis, representing close to half of patients.
Neither pill is cheap. Although Kalydeco was hailed as a miracle treatment, one doctor called its initial $300,000-a-year price tag “unconscionable” (see “A Tale of Two Drugs”). Orkambi costs less, but its benefits are not quite as clear.
In Holland, where Kalydeco is on sale, organoids have already been used to determine whether it should be prescribed for patients with ultra-rare mutations never studied in any trial. One patient, a teen named Fabian, had his case featured on the popular Dutch talk show The World Goes On. (Here’s a video describing his case.) Fabian’s gene mutation was unique, so it had never been studied. But researchers made an organoid for him and showed that Kalydeco worked. “Our organoid showed he would benefit,” says Clevers. Once Fabian began swallowing the pills, “he got better in hours.”
Vertex thinks identifying more people who can benefit from the drugs is the correct role for organoids. The company says there are now several cases in Holland where insurers are paying for Kalydeco because of organoid results.
But now the debate is over Orkambi—and the question is whether organoids can be used to limit the drug’s use, not expand it.
The drug is approved by regulators, but its price (about $180,000 in Europe) remains an obstacle for a small country like the Netherlands. According to a report prepared for the Dutch health ministry by Zorginstituut Nederland, an institute that advises the state on cost-effectiveness, providing it to all eligible patients would cost $120 million a year, an expense deemed unreasonable since many patients wouldn’t actually be helped.
The institute suggested that the drug was worth about 82 percent less than what Vertex was asking. Soon enough, negotiations between Vertex and the health ministry broke down. “Somehow they didn’t meet in the middle, and this was really, really bad news for us,” says Jacquelien Noordhoek, who leads the largest group representing Dutch cystic fibrosis patients. “The price is too high, I agree.”
Unlike Vertex, Dutch scientists and patient organizations say that even among individuals with the correct mutation, it’s apparent the drug doesn’t work for everyone. If every patient had an organoid made, says Clevers, the drug could be dispensed only to those whose organoids responded. That could make the treatment affordable at a national level, despite its sticker price.
The catch-22 is that in order to run such a study, the Dutch government first has to agree to pay for the drug so it’s available to patients in the first place. That’s why Clevers and Noordhoek were at the ministry last week pressing for its authorization.
Keep Reading
Most Popular
Large language models can do jaw-dropping things. But nobody knows exactly why.
And that's a problem. Figuring it out is one of the biggest scientific puzzles of our time and a crucial step towards controlling more powerful future models.
The problem with plug-in hybrids? Their drivers.
Plug-in hybrids are often sold as a transition to EVs, but new data from Europe shows we’re still underestimating the emissions they produce.
Google DeepMind’s new generative model makes Super Mario–like games from scratch
Genie learns how to control games by watching hours and hours of video. It could help train next-gen robots too.
How scientists traced a mysterious covid case back to six toilets
When wastewater surveillance turns into a hunt for a single infected individual, the ethics get tricky.
Stay connected
Get the latest updates from
MIT Technology Review
Discover special offers, top stories, upcoming events, and more.