How Much Force Do Bacteria Produce? It’s Now Been Measured
Bacteria migrate using a range of curious ambulatory mechanisms. These migrations allow them to follow prey, to form biofilms, and simply to aggregate.
And that raises a curious question. Given this ability to move, how much force do bacteria produce as they go? In other words, how hard can they push?
Today we get an answer thanks to the work of Joshua Shaevitz, Benedikt Sabass, and Howard Stone at Princeton University. These guys have developed a method for measuring the tiny forces involved and show that when it comes to push and shove, bacteria punch well above their weight.
A typical bacterial cell is just a few micrometers in length and has a mass in the region of 10-15 kilograms. Under the force of gravity, a single cell would exert a force of around 10 femtonewtons. That’s not an easy force to measure.
Shaevitz and co attempt it using a technique known as traction force microscopy. This is based on the observation that bacteria deform any soft material around them as they move. So by measuring these deformations, it is possible to calculate the forces behind them.
The experiment involves placing the bacteria on a soft gel-like material and then using a microscope to photograph them as they move. The material in question is a thin layer of soft elastic gel made of chitosan-coated polyacrylamide. This has well-characterized material properties which makes it straightforward to calculate how much force is required to deform it.
But when deformations are small, they are hard to see. So the gel also contains microbeads of two different colors that move as the material deforms and are easier to see. As cells move across the surface, any change in position of the microbeads can be used to calculate the deformations that this movement causes.
Shaevitz and co carry out their experiments on Myxococcus xanthus bacteria, which move using two different mechanism. The first is a kind of gliding motion in which the cell membrane in contact with the surface acts like a tank track as the creature moves. A single gliding cell produces forces of just a few piconewtons (10-12 Newton), which is hardly enough to deform the gel. “We conclude that gliding of individual cells is a low-friction process that hardly affects the environment mechanically,” say Shaevitz and co.
However, Myxococcus xanthus have another, more powerful way of moving. This is a kind of grapple-hook mechanism in which each cell produces small hair-like protuberances called a pili which reach ahead and attach to the surface. By reeling in the pili, the bacteria pull themselves along at speeds of around one micrometer per second, or about one body length per second.
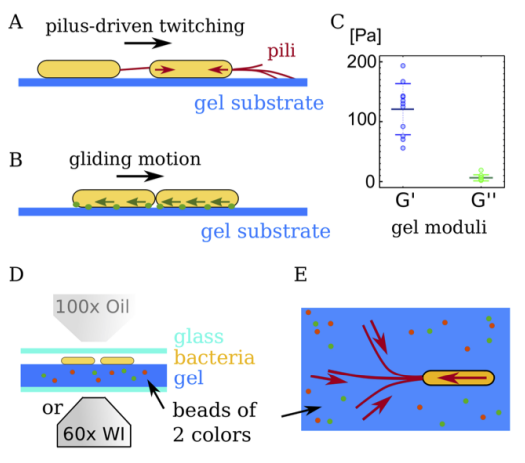
In this case, Shaevitz and co say the average force generated by a single cell is about 50 piconewtons—that’s 10 times higher than for gliding motion.
What’s more, bacteria generally move in groups, so their collective forces can be much higher. The measurements show that groups of bacteria exert a force of more than 100 piconewtons.
That’s interesting work that reveals at least some of the capabilities of bacteria as locomotive machines.
There are still significant unanswered questions, however. For example, the resolution of this kind of traction force microscopy is about 0.5 micrometers, meaning that deformations smaller than this cannot be measured. So this technique misses any dynamics that occur on a smaller scale.
There are also plenty of other mysteries associated with bacterial movement. For example, nobody knows why Myxococcus xanthus can move faster on soft agar than on stiff agar. But this kind of work should help reveal answers.
Beyond this, an interesting question is how to exploit bacterial movement. If this movement generates forces, why not use them to push levers, operate switches, turn hamster wheels, carry cargo, and so on? It’s not hard to imagine a veritable Disneyland of bacterial activity.
Of course, machinery at this scale operates in an entirely different way to the human scale—inertial forces become insignificant while other effects such as van de Waal’s forces become hugely important. That’s something designers of microelectromechanical devices have long known—perhaps they could help?
Indeed, it’s not beyond imagination that the collective forces of migrating bacteria might one day be harnessed to carry out useful work on the micrometer scale.
Ref: arxiv.org/abs/1701.00524: Collective Force Generation by Groups of Migrating Bacteria
Keep Reading
Most Popular
Large language models can do jaw-dropping things. But nobody knows exactly why.
And that's a problem. Figuring it out is one of the biggest scientific puzzles of our time and a crucial step towards controlling more powerful future models.
The problem with plug-in hybrids? Their drivers.
Plug-in hybrids are often sold as a transition to EVs, but new data from Europe shows we’re still underestimating the emissions they produce.
Google DeepMind’s new generative model makes Super Mario–like games from scratch
Genie learns how to control games by watching hours and hours of video. It could help train next-gen robots too.
How scientists traced a mysterious covid case back to six toilets
When wastewater surveillance turns into a hunt for a single infected individual, the ethics get tricky.
Stay connected
Get the latest updates from
MIT Technology Review
Discover special offers, top stories, upcoming events, and more.