In First Human Test of Optogenetics, Doctors Aim to Restore Sight to the Blind
If all goes according to plan, sometime next month a surgeon in Texas will use a needle to inject viruses laden with DNA from light-sensitive algae into the eye of a legally blind person in a bet that it could let the patient see again, if only in blurry black-and-white.
The study, sponsored by a startup called RetroSense Therapeutics, of Ann Arbor, Michigan, is expected to be the first human test of optogenetics, a technology developed in neuroscience labs that uses a combination of gene therapy and light to precisely control nerve cells.
The trial, to be carried out by doctors at the Retina Foundation of the Southwest, will involve as many as 15 patients with retinitis pigmentosa, a degenerative disease in which the specialized light-sensitive photoreceptor cells in the eye die, slowly causing blindness. The aim of the treatment is to engineer the DNA of different cells in the retina, called ganglion cells, so that they can respond to light instead, firing off signals to the brain.
The Texas study will be followed closely by neuroscientists who hope to eventually use optogenetics inside the human brain to treat Parkinson’s or severe mental illness. “This is going to be a gold mine of information about doing optogenetics studies in humans,” says Antonello Bonci, a neuroscientist who is scientific director of the intramural research program at the National Institute on Drug Abuse in Baltimore.
Patients who have retinitis pigmentosa lose peripheral and night vision before eventually becoming blind. Candidates for the RetroSense study won’t be able to see much more than a hand moving in front of their face. RetroSense CEO Sean Ainsworth says he hopes that after the treatment patients will “see tables and chairs” or maybe read large letters.
Optogenetics was developed a decade ago in neuroscience labs as a way to precisely control the activity of nerve cells. It works by adding DNA instructions for a light-sensitive protein, channelrhodopsin, that algae use to sense sunlight and move toward it. Added to a nerve, it causes the cell to fire when exposed to a specific wavelength of light.
The technology is already helping scientists make rapid progress in understanding what brain cells underlie movement, motivation, pain, and many other basic brain functions in animals. In one experiment, Stanford University researchers led by Karl Deisseroth, one of the inventors of optogenetics, found they could switch the sensation of fear on and off in mice by shooting light through a fiber-optic cable at specific cells in their brains.
RetroSense was founded in 2009 to commercialize research carried out by Zhuo-Hua Pan, a Wayne State University vision expert who realized that the eye might be the easiest place to use optogenetics. Unlike the brain, the eye is transparent and sensitive to light, and it’s much easier to treat with gene therapy. No extra hardware or fiber-optic cables are needed, since light shines directly onto the retina.
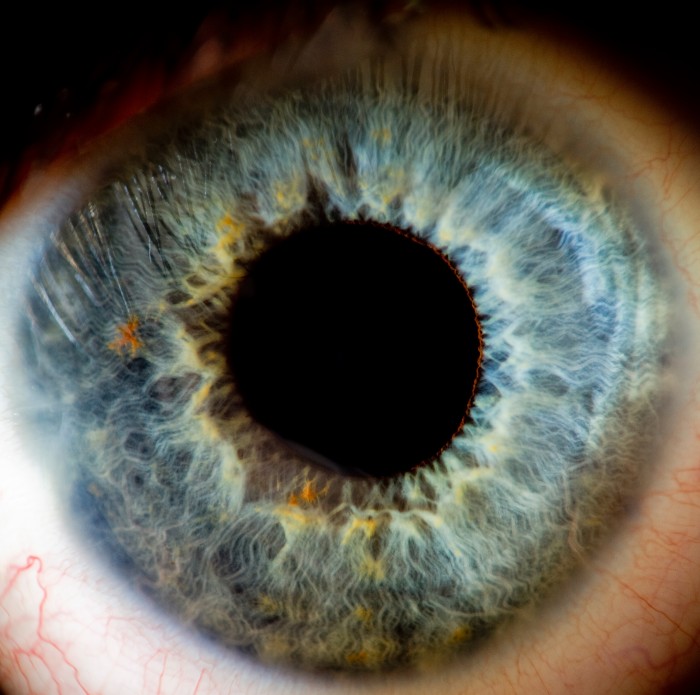
The eye has two kinds of photoreceptor cells. Cones, named for their shape, are responsible for color vision. Rods respond to light at night. Both react to incoming photons by generating an electrical signal that is passed through a succession of nerve cells to the optic nerve and then to the brain.
To overcome the loss of photoreceptors, the strategy created by Pan and adopted by RetroSense works by injecting viruses laden with algae DNA into the center of the eye. Their target is the topmost layer of cells in the retina, called ganglions. Once they start making the light-sensitive protein, the ganglion cells should fire in response to light.
Pan expects the treatment to generate at least 100,000 light-sensing cells in the retina. That could translate to substantial vision. So far, the only commercial technology to restore limited sight to blind people is an electrical implant called the Argus II that transmits video from a camera to a sheet of 60 electrodes stitched inside the retina, but it provides only a few pixels of visual information at a time.
The algae protein has some limitations. One is that it responds only to the blue component of natural light. As a result, RetroSense expects patients to experience monochromatic vision. Perhaps the brain will process this as black and white, says Ainsworth. Patients might perceive an object that doesn’t reflect any blue light at all as being black.
Speculation about what people will or won’t see—and what that subjective experience will be like—stems from results of studies on blind mice. Jens Duebel, who leads a group studying optogenetic vision restoration at the Institut de la Vision, in Paris, says that after treatment blind mice will move their heads to follow an image and also avoid a bright light when held in a dark box, just as healthy mice do.
Because the algae protein isn’t as sensitive to light as a normal retina, Duebel thinks patients might see in outdoor light but not very well indoors. Duebel is associated with GenSight Biologics of Paris, a company that developed a pair of goggle-mounted microprojectors it thinks could overcome that problem. The goggles will convert a video feed into wavelengths of light that a genetically altered retina can respond to. The French company remains a few years away from starting a clinical trial of its technology, Duebel says.
Other treatments using optogenetics are under development. A California company, Circuit Therapeutics, is developing an optogenetic treatment for chronic pain. Circuit is also being funded by the Michael J. Fox Foundation for Parkinson’s Research, which wants to determine whether it’s possible to control Parkinson’s tremors using a light source inside the brain. Until now, this has been achieved with drugs or implanted electrodes.
Bonci says that before optogenetics can be used therapeutically in the brain, researchers will need more information about which cells to target. “But that’s five years away, not 20 years away,” he says.
Deep Dive
Biotechnology and health
How scientists traced a mysterious covid case back to six toilets
When wastewater surveillance turns into a hunt for a single infected individual, the ethics get tricky.
An AI-driven “factory of drugs” claims to have hit a big milestone
Insilico is part of a wave of companies betting on AI as the "next amazing revolution" in biology
The quest to legitimize longevity medicine
Longevity clinics offer a mix of services that largely cater to the wealthy. Now there’s a push to establish their work as a credible medical field.
There is a new most expensive drug in the world. Price tag: $4.25 million
But will the latest gene therapy suffer the curse of the costliest drug?
Stay connected
Get the latest updates from
MIT Technology Review
Discover special offers, top stories, upcoming events, and more.