Designer Carbons Are Getting a Boost from Nanotechnology
A group of Stanford researchers have come up with a nanoscale “designer carbon” material that can be adjusted to make energy storage devices, solar panels, and potentially carbon capture systems more powerful and efficient.
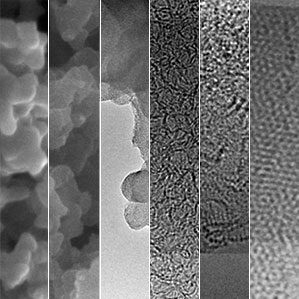
The designer carbon that has reached the market in recent years shares the Swiss-cheese-like structure of activated carbon, enhancing its ability to catalyze certain chemical reactions and store electrical charges; but it’s “designed” in the sense that the chemical composition of the material, and the size of the pores, can be manipulated to fit specific uses.
The designer carbon tested at Stanford is “both versatile and controllable,” according to Zhenan Bao, a professor of chemical engineering and the senior author of the study, which appeared in the latest issue of the journal ACS Central Science.
“Producing high-surface-area carbons with controlled chemical composition and morphology is really challenging,” says Bao. Other methods currently available, she says, “are either quite expensive or they don’t offer control over the chemical structure and morphology.”
The work of Bao and her team represents the latest step forward in a rapidly advancing field with huge promise for a variety of clean-tech applications. Seattle-based EnerG2, for example, has pioneered designer carbons in several applications, particularly lithium-ion batteries. Replacing graphite in the batteries’ anode with designer carbon has resulted in dramatic performance improvements—up to a 30 percent increase in the batteries’ storage capacity, according to EnerG2 founder and CTO Aaron Feaver.
Essentially, designer carbon material is created by baking a precursor material at very high temperatures and then chemically treating it to produce a porous 3-D structure with an enormous surface area. The Stanford team began with a complex polymer that forms an interconnected framework. The cooking temperature can be adjusted, from 300 ◦C up to 900 ◦C, to fine-tune the material’s properties. The result is sheets of carbon that are as little as one nanometer thick, with more than 4,000 square meters of surface area per gram.
Designer carbon usually costs more than other anode materials, particularly graphite, but Bao says the raw materials for the Stanford experiments cost less than $10 for every kilogram of carbon produced.
Two of the most promising applications tested at Stanford are lithium-sulfur batteries and supercapacitors. Lithium-sulfur batteries have several advantages over conventional lithium-ion systems, but also one serious flaw: they tend to leak lithium polysulfide, causing the battery to fail. The nano-sized pores of the designer carbon prevent that from happening.
Supercapacitors are energy-storage devices that charge and discharge at very high rates. Bao’s team found that supercapacitor electrodes with the new carbon had electrical conductivity three times that of ones with conventional activated carbon.
Another form of designer carbon is carbon nanotubes, which could be suitable for a number of applications including energy storage. In 2012, MIT scientist Joel Schindall unveiled a method of growing carbon nanotube “forests” that could produce supercapacitors whose energy-storage capacity rivals that of batteries.
The most far-reaching application for designer carbon, though, would be in carbon capture—a technology that has been talked about for years but has never been economically viable. Designer carbon material with pores of a certain size shows great promise in selecting for carbon dioxide, which would condense onto the surface of the synthetic carbon in a capture system.
“Our synthetic approach gives us a very versatile, tunable material, and we are developing a different version of the carbon that’s highly suitable for carbon capture,” says Bao. That work has just been submitted for publication, she adds.
Editor’s note: This story has been updated to clarify the nanoscale carbon advancement from Stanford.
Keep Reading
Most Popular
Large language models can do jaw-dropping things. But nobody knows exactly why.
And that's a problem. Figuring it out is one of the biggest scientific puzzles of our time and a crucial step towards controlling more powerful future models.
The problem with plug-in hybrids? Their drivers.
Plug-in hybrids are often sold as a transition to EVs, but new data from Europe shows we’re still underestimating the emissions they produce.
Google DeepMind’s new generative model makes Super Mario–like games from scratch
Genie learns how to control games by watching hours and hours of video. It could help train next-gen robots too.
How scientists traced a mysterious covid case back to six toilets
When wastewater surveillance turns into a hunt for a single infected individual, the ethics get tricky.
Stay connected
Get the latest updates from
MIT Technology Review
Discover special offers, top stories, upcoming events, and more.