A Second Artificial Retina Option for the E.U.
A sight-restoring implant approved for sale in the European Union on Wednesday is the second artificial retina to become available in the region.
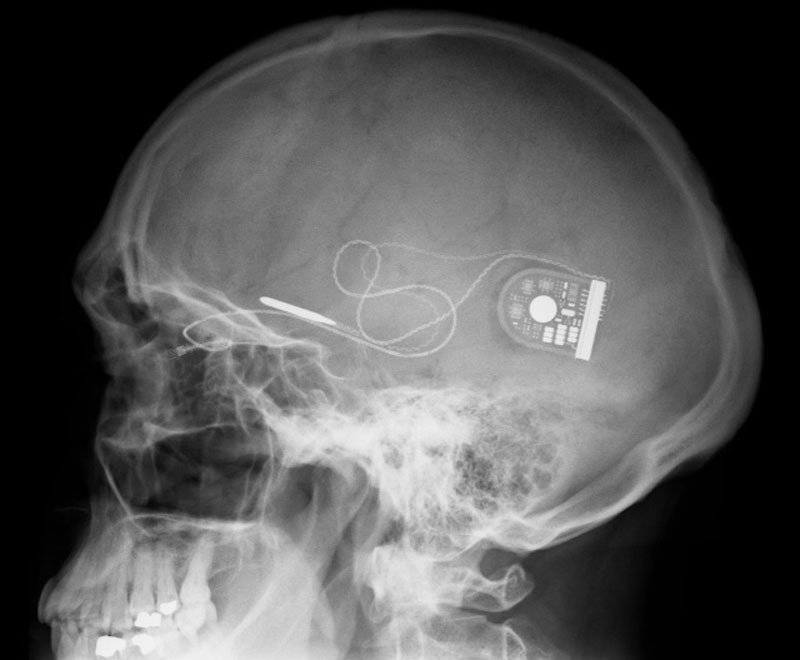
Germany’s Retina Implant developed the device, called Alpha IMS, which features a light-sensitive three-millimeter square microchip that detects images with photodiodes and communicates the information electrically to nerve cells in the retina (see “Microchip Restores Vision”). The device has been approved to treat patients blinded by a degenerative eye condition called retinitis pigmentosa, which causes the rods and cones of the retina to die over time.
Another artificial retina system, made by a California company, has been available in Europe since 2011 (see “Artificial Vision”). This system, the Argus II, from Second Sight uses a camera mounted on spectacles to detect light and communicates that information to an implant in the retina. The Alpha IMS, conversely, requires no externally visible gear. Each system also requires a different kind of surgery for implantation in the retina—the surgery for the Argus II takes about 3 hours, whereas the surgery for the Alpha IMS takes up to 10.
The vision restored by both technologies is far from complete and varies widely from patient to patient (see “What It’s Like to See Again with an Artificial Retina”). Some report being able to see slow-moving cars, open doorways, and household objects, while others don’t experience any improvement. Still, experts are hopeful that these early versions of retina prostheses will one day be replaced by better models, which will likely require, among other improvements, more electrodes to pass along image information to the brain.
According to Retina Implant’s CEO Walter-G Wrobel, the cost of the Alpha IMS device and surgery is approximately 100,000 Euros (around $130,000). The company is currently pursuing approval by the Food and Drug Administration to begin clinical trials in the U.S.
Keep Reading
Most Popular
Large language models can do jaw-dropping things. But nobody knows exactly why.
And that's a problem. Figuring it out is one of the biggest scientific puzzles of our time and a crucial step towards controlling more powerful future models.
How scientists traced a mysterious covid case back to six toilets
When wastewater surveillance turns into a hunt for a single infected individual, the ethics get tricky.
The problem with plug-in hybrids? Their drivers.
Plug-in hybrids are often sold as a transition to EVs, but new data from Europe shows we’re still underestimating the emissions they produce.
Google DeepMind’s new generative model makes Super Mario–like games from scratch
Genie learns how to control games by watching hours and hours of video. It could help train next-gen robots too.
Stay connected
Get the latest updates from
MIT Technology Review
Discover special offers, top stories, upcoming events, and more.