Making Spider-Strength Materials
Researchers have been trying to make artificial spider silk–a lightweight, tougher-than-steel material that could have countless industrial applications–for decades. In an important step toward that goal, researchers at Tufts University have created genetically engineered microbes that produce more of the proteins needed to make spider silk than ever before.
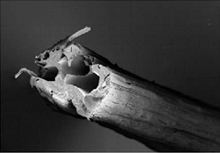
Dragline silk–the type spiders use for the rims and spokes of their webs–is tougher and far lighter than steel. Engineered bacteria can produce the proteins needed to synthesize this silk, which is spun together to make fibers. However, previous efforts to make spider silk using bacteria have been hamstrung for several reasons. First, researchers have had an incomplete picture of the dragline silk gene sequence. And second, they’ve had limited success in modifying the bacteria to produce enough of the proteins.
David Kaplan, chair of the biomedical engineering department at Tufts University, has pioneered the application of silkworm silk in medical devices, biodegradable electronics, optical devices, and adhesives. He believes that spider silk, which is stronger than the silkworm variety, could open up new applications, but says, “It hasn’t been explored as much because we haven’t had enough material.” Spiders are aggressive and territorial and thus can’t be farmed like silkworms.
Bioengineers have had only modest success in getting microbes to make spider-silk proteins. Chemical giant DuPont tried unsuccessfully to develop a bacteria-produced silk product in the 1990s. Part of the problem is that spider silk is made from a very large protein with a highly repetitive genetic sequence, making it hard to decode, says Christopher Voigt, professor of pharmaceutical chemistry at the University of California, San Francisco.
Last year, researchers using new sequencing technologies produced the first complete genetic sequence for spider silk. Before that, researchers were forced to use truncated silk genes, and fibers made using these genes were not as strong and tough as natural silk.
Even with the full dragline silk gene sequence, producing artificial silk is a challenge. Making enough of the protein requires a larger amount of starting material than the bacteria naturally contain. Working with researchers at the Korea Advanced Institute of Science and Technology in Daejeon and Seoul National University, Kaplan added the full silk gene to E. coli and then altered the bacteria’s protein-making pathway so that it makes sufficient quantities of the amino acids needed to enable silk production. Previously, engineered bacteria have only been able to produce tens of milligrams of the protein per liter. Kaplan’s E. coli yield one to two grams per liter.
“They’ve clearly shown that E. coli can make these large proteins, and engineered them to have the resources to do it,” says Randy Lewis, professor of molecular biology at the University of Wyoming. Lewis predicts that it will be possible to use a bacterial system to produce kilogram quantities of artificial spider silk within a few years.
Kaplan says that’s his plan. “We’d like to turn it into a continuous production process,” he says.
Kaplan says what’s needed now are more energy-efficient methods for making the proteins into fibers. Using spinning methods similar to those used to make polymer fibers such as polyester, his group has created fibers from the team’s proteins with properties comparable to natural dragline silk in terms of strength, elasticity, and toughness. However, because spider-silk proteins are finicky and insoluble in water, spinning them into fibers requires high-temperature processing and harsh solvents.
The fibers “take a huge amount of energy to put together,” says Kaplan. Materials scientists would like to make silk fibers the way spiders do: at ambient temperatures, with no harsh solvents.
A novel approach to the problem is being pursued by Luke Lee, director of the molecular nanotechnology center at the University of California, Berkeley. He is designing spinning systems that incorporate microfluidic channels designed to provide the salt- and solvent- gradients found in spider glands. A company called Refactored Materials, founded by students of Lee’s and Voigt’s, is also working on the spinning problem.
Keep Reading
Most Popular
Large language models can do jaw-dropping things. But nobody knows exactly why.
And that's a problem. Figuring it out is one of the biggest scientific puzzles of our time and a crucial step towards controlling more powerful future models.
The problem with plug-in hybrids? Their drivers.
Plug-in hybrids are often sold as a transition to EVs, but new data from Europe shows we’re still underestimating the emissions they produce.
Google DeepMind’s new generative model makes Super Mario–like games from scratch
Genie learns how to control games by watching hours and hours of video. It could help train next-gen robots too.
How scientists traced a mysterious covid case back to six toilets
When wastewater surveillance turns into a hunt for a single infected individual, the ethics get tricky.
Stay connected
Get the latest updates from
MIT Technology Review
Discover special offers, top stories, upcoming events, and more.