Patients’ Social Network Predicts Drug Outcomes
Earlier this month, the journal Lancet Neurology published a study showing that the generic drug lithium did nothing to slow the course of amyotrophic lateral sclerosis (ALS), a devastating neurological disease. The findings would likely have been a disappointment to patients–they refuted an earlier, much smaller study suggesting that lithium could alter the disease’s rapid decline–but many already suspected this outcome. Eighteen months earlier, PatientsLikeMe, a for-profit patient networking site and data aggregator based in Cambridge, MA, had come to a similar conclusion, much more quickly and at much less cost.

The site, part social networking and part health 2.0, has gathered a wealth of data on its 65,000 members, which span 16 different disease communities, including epilepsy, fibromyalgia, and depression. It provides users with tools to track their health status and communicate with other patients, and then removes the personal details and sells the data to pharmaceutical companies and others. The company’s cofounder, James Heywood, believes the site will ultimately change the way drugs and other interventions are evaluated. Heywood, his brother Ben, and a former MIT classmate, Jeff Cole, founded PatientsLikeMe in 2006 as a way to help a third brother, Stephen, who was diagnosed with ALS in 1998.
The approach won’t replace clinical trials, at least anytime soon. But some experts do believe it could have enormous benefits, highlighting how different types of patients use drugs, when they stop, or what side effects they experience. “The beauty of observational trials is that you can see how an intervention works in the real world,” says Mark Roberts, a physician and professor of Health Policy and Management at the University of Pittsburgh. For example, many trials eliminate patients with secondary ailments, such as renal failure or chronic obstructive pulmonary disorder. “All my patients have those things, so how do I know it works in people I see?” he says.
PatientsLikeMe put its database to the test in 2008, after a small Italian study published in Proceedings of the National Academy of Sciences suggested that lithium could delay the progression of ALS. About 10 percent of PatientsLikeMe’s ALS users began taking the drug, not wanting to wait for a larger trial to confirm the results. Inspired by a member in Brazil who wanted to know if lithium was truly helping, the company rolled out a number of tools to allow patients to track their progress.
The founders, who trained as engineers at MIT, began building models of how the disease typically progressed in individuals with certain characteristics, incorporating variables such as age, gender, disease severity, time since diagnosis, and other factors. Heywood says the models allow researchers to predict the course of an individual’s disease more accurately than the standard prognostic tools. “We can predict when a patient will die 16 months ahead of time, compared to the typical doctor report of ‘you have two to five years to live,’ ” he says.
Because the company had such extensive data on the patients, researchers could analyze how an individual’s symptoms changed 12 months before they began taking lithium, as well as after. Unlike a typical clinical trial, this allowed scientists to search for unique characteristics in the people who decided to take the drug. They found that people who chose to take it were somewhat worse off before starting the drug than those who didn’t. (This group may have been more motivated to try an experimental treatment.)
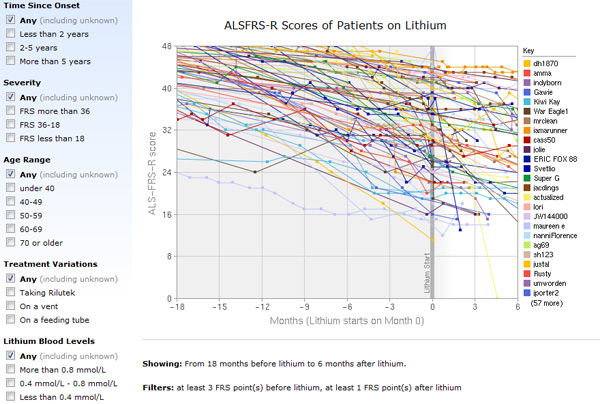
The researchers could also compare lithium takers to controls in a more nuanced way. By compiling data from patients with similar backgrounds and disease characteristics who did not take lithium, they created a model predicting the course of the disease in that group. They could then determine whether a patient who fit those criteria pretreatment deviated from that progression after taking the drug. The answer was no, meaning lithium had no effect–positive or negative–on the disease.
Criticisms of the approach mimic those typically made of observational trials, which lack a “blind” placebo control group. Outside of a controlled clinical trial, it’s difficult to determine whether the drug or some other factor was the key to the outcome. “The problem with PatientsLikeMe is that it involves observational information. If patients think medication is helping, they will be biased toward recognizing whatever positive events they have and vice versa,” says Paul Bleicher, founder of PhaseForward, a clinical trial data-management company. “Most people aren’t even aware of the bias. That’s why blind trials exist.”
University of Pittsburgh’s Roberts concurs. “What kinds of patients are willing to report their data? Is it the full range of disease? Were people who didn’t do well as likely to report findings as those who didn’t?” he asks. However, Roberts is also optimistic, pointing out that statistical methods can correct for many of these issues. “As long as you are really careful about understanding the possible biases, I think you can begin to approximate the control you have in clinical trials,” he says.
“The types of things you can get from observational studies are the generation of valuable hypotheses, which is not easy to do,” says Bleicher, who also works for Humedica, a health-care informatics company that collects data from electronic medical records. “Using databases, you can come up with observations you believe are strong enough to be worthy of doing a controlled trial.”
Swati Aggarwal, a physician at Massachusetts General Hospital in Boston who led the Lancet study on lithium and ALS, sees PatientsLikeMe as a rich resource for accessing the ALS community. “We could use the database to try to understand why patients don’t like to use BPAP” (bilevel positive airway pressure), a ventilator to help patients breathe, she says.
PatientsLikeMe is currently building models for its other disease communities and next plans to look at the effects of some treatments for multiple sclerosis, as well as nondrug factors in ALS. “The diseases we focus on tend to be those with patients who know more about their health than the medical community does,” says Heywood. “It’s easier to get patients to tell us than to get medical systems to change.”
Keep Reading
Most Popular
Large language models can do jaw-dropping things. But nobody knows exactly why.
And that's a problem. Figuring it out is one of the biggest scientific puzzles of our time and a crucial step towards controlling more powerful future models.
The problem with plug-in hybrids? Their drivers.
Plug-in hybrids are often sold as a transition to EVs, but new data from Europe shows we’re still underestimating the emissions they produce.
Google DeepMind’s new generative model makes Super Mario–like games from scratch
Genie learns how to control games by watching hours and hours of video. It could help train next-gen robots too.
How scientists traced a mysterious covid case back to six toilets
When wastewater surveillance turns into a hunt for a single infected individual, the ethics get tricky.
Stay connected
Get the latest updates from
MIT Technology Review
Discover special offers, top stories, upcoming events, and more.