How Fish Grow New Hearts
The small, unassuming zebrafish, which has become a stable in biology labs across the globe, can perform an impressive feat of regeneration–it can withstand losing 20 percent of a ventricle, a chamber of the heart, growing it back within a month. Two new studies published yesterday in Nature show the animals regrow their hearts by triggering cell division of adult heart muscle cells rather than via stem cells. If researchers can elucidate the chemical signaling involved in the process, they may be able to find ways to stimulate heart repair or regeneration in humans. While recent research suggests that human hearts do have a limited capacity to generate new cells, heart muscle tends to form scars after heart attack rather than healthy new tissue.

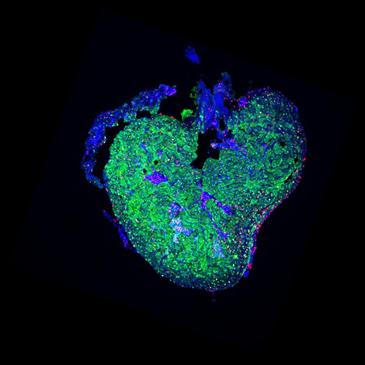
Researchers in Barcelona and San Diego developed zebrafish whose heart muscle cells glow green. After cutting away 20 percent of the fish’s ventricle, researchers found that the new replacement cells also glowed green, suggesting they arose from differentiated adult cells rather than cardiac stem cell. According to ScienceNow,
…Further experiments showed that the cardiomyocytes near the injury site seem to take a step backward in development, detaching from one another and losing their typical shape–presumably to make it possible for them to start dividing again as they replenish the lost tissue.

In a second study, researchers from North Carolina engineered heart cells to glow green when they express a protein unique to embryonic heart cells. Injury to an animal’s heart triggered the green glow in nearby cells, suggesting they were de-differentiating in preparation for division. Both studies were published in Nature.
According to an article in the New York Times,
Charles Murry, an expert on heart cell biology at the University of Washington in Seattle, said the two reports raised the tantalizing question of why human hearts could not complete the regeneration process. In human hearts, too, Dr. Murry said, the muscle cells dedifferentiate after injury and double up their DNA, a necessary precursor to cell division. But they do not finish the process, for reasons that are so far unknown.
Learning how to overcome that block may not be so easy, in Dr. Murry’s view. “It’s tempting to say ‘Let’s do it how nature does it,’ ” he said, “but we don’t know how nature does it. Some of the best molecular biologists in the world have been working on this for a couple of decades and it hasn’t cracked yet.”
Keep Reading
Most Popular
Large language models can do jaw-dropping things. But nobody knows exactly why.
And that's a problem. Figuring it out is one of the biggest scientific puzzles of our time and a crucial step towards controlling more powerful future models.
The problem with plug-in hybrids? Their drivers.
Plug-in hybrids are often sold as a transition to EVs, but new data from Europe shows we’re still underestimating the emissions they produce.
Google DeepMind’s new generative model makes Super Mario–like games from scratch
Genie learns how to control games by watching hours and hours of video. It could help train next-gen robots too.
How scientists traced a mysterious covid case back to six toilets
When wastewater surveillance turns into a hunt for a single infected individual, the ethics get tricky.
Stay connected
Get the latest updates from
MIT Technology Review
Discover special offers, top stories, upcoming events, and more.