Xudong Wang
Powering the nanoworld.
When Xudong Wang finished his PhD in materials science at Georgia Tech at the end of 2005, he knew he had a good thing going. He opted to stay put in the lab of Zhong Lin Wang (no relation), sure that he and his lab mates were close to creating a new nanotech-based generator–an invention they felt could change the future of nanotechnology.
His risk paid off earlier this year when Science published a paper he coauthored, describing a novel device that converts ultrasonic waves–high-frequency mechanical vibrations–into electricity. The tiny device turns out a steady 0.5 nanoamperes of current that engineers may one day be able use to power implantable biosensors, remote environmental monitors, and more. “It’s a very cool concept,” says Peidong Yang, a nanowire researcher at the University of California, Berkeley. “Vibrational energy is everywhere.” If Wang’s devices can harness it cheaply, “the impact could be big,” Yang says.
The generator is the culmination of several remarkable advances made by Wang since he arrived in Z. L. Wang’s lab from China in 2002. Others had made nanowires of zinc oxide (ZnO), a versatile optical, semiconductor, and piezoelectric material, but the production process typically left them tangled like spaghetti. Many prospective uses of nanowires, however, require that they form an orderly array. By 2004, Xudong had found a way to use gold to catalyze the emergence of an organized forest of wires from a vapor of zinc oxide dust.
While Xudong was finishing up his PhD, Z. L. Wang and Jinhui Song, another graduate student in the lab, showed that they could generate a tiny electric current by bending individual ZnO nanowires with the tip of an atomic force microscope. Still, to make practical energy harvesters, the researchers needed a way to collect energy from thousands of nanowires flexing simultaneously.
They began with one of Xudong Wang’s miniature ZnO forests, grown atop an electrode made from gallium nitride, sapphire, or a conducting polymer. Xudong capped this with a second electrode made of platinum-coated silicon and studded with parallel rows of tiny peaks and trenches, like lines of saw teeth. He then used ultrasound waves to vibrate the electrodes. The motion squeezed the two electrodes together, causing the nanowires between them to flex and generate a current; the current flowed through the platinum coating and into an external circuit.
Conceiving the generator was a group effort, but Z. L. Wang gives Xudong credit for pulling off the demonstration. “Anything you can think up, he can make it work,” Z. L. says.
The two-square-millimeter devices turn out just a trickle of power, but in the months since its Science paper appeared, the team has already boosted the devices’ output current 30-fold. And there’s plenty of room for improvement: ensuring that all the nanowires are actively generating current, for example, could boost the power output to as much as four watts per cubic centimeter. “If we do that, we can power portable electronics such as cell phones,” Xudong says. The group is also trying to make versions of the device that generate current in response to lower-frequency sound waves and to mechanical vibrations. That could allow nanotechnologists to harvest energy anywhere, from the interior of a pulsing blood vessel to the chassis of a car rattling down the highway.
David Berry
Renewable petroleum from microbes
David Berry is sitting in a midtown Manhattan coffee shop, taking a break from a carbon-trading conference across the street, when a news report on the wall-mounted television catches his eye. The CNN dispatch describes how scientists have shown, in animal experiments, that Viagra might be used to alleviate symptoms of jet lag.
“It’s interesting,” Berry says, chuckling, as his eyes wander back to the screen. “We were talking about a year ago of using Viagra to treat jet lag.” One side effect of Viagra widely reported in the medical literature has been the perception of blue light, he continues, and blue light has also been shown to reset circadian clocks in humans. “I like when I see these things actually come true,” he says.
It’s one thought that never went beyond a blue-sky conversation among his venture capital colleagues. But it reflects how easily ideas come to Berry, a Harvard-trained MD who earned his PhD through the Biological Engineering Division at MIT and for the past two years has been a principal in the venture capital firm Flagship Ventures in Cambridge, MA.
Since receiving his bachelor’s degree from MIT in 2000, Berry has helped develop a way to treat stroke, thought up a new approach to cancer therapy, and, most recently, created a system to genetically engineer microbes to produce biofuels. He has 21 patent applications pending, and his intellectual curiosity touches on therapeutic medicine, diagnostic devices, and now, most notably, alternative energy technologies. His innovations in energy form the conceptual basis of LS9, a California-based renewable-petroleum company that has received $5 million in venture funding from Flagship and Khosla Ventures in California (see “Better Biofuels,” July/August 2007).
Berry points out that a number of the pioneering biotech companies were thinking about energy and biofuels, specifically ethanol, in the 1970s. “What’s interesting,” he says, “is that, as a field, we’re making a full circle and going back to the things biotechs thought about way back then. But now we’re bringing new technological tools to make the same problems more tractable.”
On the first working day of each week, Flagship Ventures holds a group meeting to review investments and discuss new ideas. One day this May, David Berry was the youngest, and probably the most earnest, of about a dozen VCs gathered in Flagship’s seventh-floor conference room, with its grand view of sailboats plying the Charles River. The meeting ran a little long, and Berry apologized when he finally emerged. “We were talking about a potential new idea in drug delivery,” he explained. Although the details of that technology remained discreetly fuzzy, it was very clear that these are heady, palpably exciting conversations for him. “You’re discussing some of the hottest, most compelling new technologies around,” he says. “I’m having a blast.”
Berry took a seat at that conference table with no formal training in finance but a track record in technology. In graduate school, he began tinkering with a molecule that could pass through the blood-brain barrier and showed promise as a stroke treatment. The protein, an engineered version of fibroblast growth factor 2, produced functional improvement in a test animal modeling symptoms of stroke, and it brought out in Berry another quality conducive to innovation: restlessness. Berry realized that studying the protein could lead to a PhD far more quickly than most projects, and he seized the occasion. He got his PhD in 2005 (finishing his MD a year later), and the biotech company ViaCell briefly attempted to develop the molecule as a drug.
Berry also experimented with ways to reversibly attach polymers to sugar molecules and came up with a way to kill cancer cells by binding polymers to heparin, the well-known blood thinner. Berry’s polymer packaging makes cancer cells absorb heparin more quickly; once inside the cells, the heparin disrupts biochemical pathways, ultimately leading to cell death. The technology garnered the attention of Momenta Pharmaceuticals, a biotech company in Cambridge, MA; Berry garnered another publication, and another patent application.
“What makes David unusual is that there’s nothing that’s going to stop him,” says Robert Langer, a chemical engineer at MIT in whose lab Berry studied. “He has no fear. He’s willing to tackle any idea, and he has lots of ideas. The breadth of his scientific curiosity and his belief in himself are pretty remarkable for somebody his age.”
In 2004, Berry had no greater ambition than to run an academic lab, develop new technologies, and hustle them out into the commercial world. But then, in 2005, Flagship Ventures sought his input on a life science company it was starting. By the end of the year, that consulting job had evolved into an invitation to join the firm as a principal. In Flagship’s emphasis on developing the core concepts for new companies in-house, Berry saw an irresistible opportunity to jump-start innovation by funding it at its earliest stages. Although Flagship’s previous startups tended to focus on traditional life sciences like genomics, the company was increasingly interested in taking biology in a new direction: energy. “Back in 2005,” Berry recalls, “we were saying, ‘What would be interesting in the fuel space?’” The project ended up in Berry’s hands.
Berry’s goal was nothing less than “to develop a novel and far-reaching solution to the energy problem.” In collaboration with genomics researcher George Church of Harvard Medical School and plant biologist Chris Somerville of Stanford University, Berry and his Flagship colleagues set out to do something that had never been attempted commercially: using the tools of synthetic biology to make microörganisms that produce something like petroleum. Berry assumed responsibility for proving that the infant company, dubbed LS9, could produce a biofuel that was renewable, better than corn-derived ethanol, and cost-competitive with fossil-based fuels.
Ethanol is the most common biofuel, but many observers, including Berry, have reservations about corn-based ethanol as a long-term solution to the fuel crisis. Ethanol has only about two-thirds as much intrinsic energy as petroleum, and producing it requires considerable agricultural resources.
Berry took the lead in designing a system that allowed LS9 researchers to alter the metabolic machinery of microörganisms, turning them into living hydrocarbon refineries. He began with biochemical pathways that microbes use to convert glucose into energy-storing molecules called fatty acids. Working with LS9 scientists, he then plucked genes from various other organisms to create a system of metabolic modules that can be inserted into microbes; in different combinations, these modules induce the microbes to produce what are, for all practical purposes, the equivalents of crude oil, diesel, gasoline, or hydrocarbon-based industrial chemicals.
Along the way, Berry and his colleagues had to soup up the activity of certain genes to increase the output of specific intermediate molecules. They also had to determine how to selectively block other metabolic pathways so that their microbes would stay focused on producing hydrocarbons. And they figured out how to make the microörganisms secrete the final product in such a way that it could easily be collected.
“David is responsible in large part for LS9’s intellectual-property real estate,” says Noubar Afeyan, Flagship’s CEO. “If you strip away his contributions, there’s no company.”
Since the technology is proprietary and still in the early stages of development, Berry won’t identify the types of organisms and the specific cellular processes involved. LS9 has been optimizing its system and trying to increase the yields of its designer biofuels at its California facility. Current yields in the lab are an order of magnitude lower than those for ethanol produced from cellulose, Afeyan says, but the company hopes to reach a comparable yield within a year.
Nonetheless, LS9 has no products so far and many hurdles to surmount. Berry’s system, for example, is designed to exploit glucose-based feedstocks such as cellulose. Berry says he is “agnostic” about what source of cellulose might drive the LS9 system on an industrial scale; he lists switchgrass, wood chips, poplar trees, and Miscanthus, a tall grass similar to sugarcane, as potential sources of biomass. But a cost-effective and efficient source of cellulose is one of the more significant bottlenecks in the production of any biofuel.
Despite these uncertainties, Berry’s method offers many of the advantages of biofuels in general. The raw feedstock would be agricultural and homegrown; it would be renewable; and it would, in principle, provide a more environmentally friendly source of energy than traditional crude oil (which requires smoke-belching refineries). With microbes doing all the work, fuels could be produced in large fermentation tanks of the sort used by biotech companies.
The biological synthesis of hydrocarbons is “a technology with really game-changing potential,” Berry says. “It has security benefits. It has sustainability benefits. And the value of that, on top of a cost benefit, makes it a very compelling technology.” And one of the most compelling parts of the story behind that technology is that it was developed by a doctor in his 20s.
Javier García-Martínez
New zeolites for cracking petroleum.
Problem: Turning crude oil into gasoline involves a process known as catalytic cracking, which splits large hydrocarbon molecules into simpler fragments. Refineries traditionally use synthetic porous materials called zeolites as catalysts for these reactions.
The standard zeolite has pores less than one nanometer across, so the largest hydrocarbon molecules can’t fit inside them and undergo the reactions that break the bonds between atoms. Increasing the pore size of the zeolites would allow a larger fraction of crude oil to be converted into useful products. Companies have spent three decades and millions of dollars trying to increase pore size, without much success.
Solution: Javier García-Martínez, leader of the Molecular Nanotechnology Lab at the University of Alicante in Spain, has developed a way to increase pore size to between two and ten nanometers, the ideal range for producing gasoline. He mixes zeolites with an alkaline solution. A soaplike surfactant is added to the solution and forms small structures that the zeolites reconstruct themselves around. The surfactant is then burned off.
Choosing surfactants of different molecular sizes allows García-Martínez to tune the pore size, so he can optimize the zeolites for other purposes, such as chemical synthesis or water treatment. He cofounded a company, Rive Technology, that is now working to commercialize the technology and test it in a working refinery.
Rachel Segalman
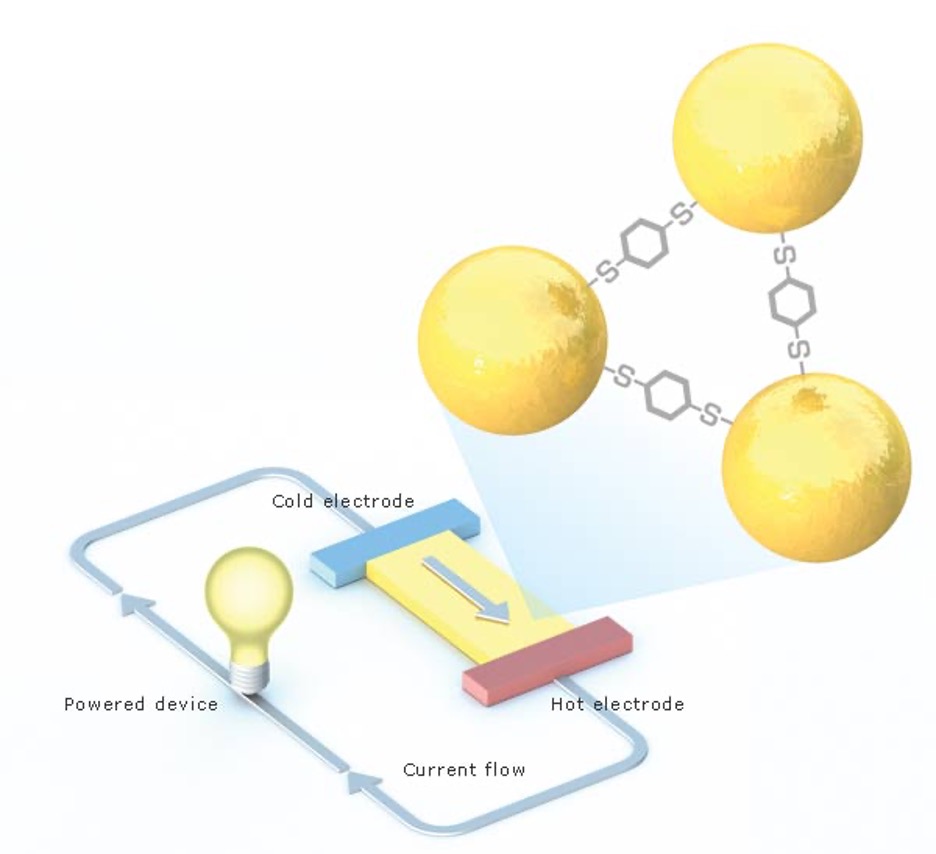
Cheap electricity from heat.
Most of the energy in fuels is wasted as heat. But much of that heat could be converted to electricity by “thermoelectric” materials–if they were cheaper and more efficient. Now Rachel Segalman, an assistant professor of chemical engineering, has discovered that cheap organic molecules can be used to generate electricity from heat. So far, the voltage produced is small, but Segalman and colleagues are modifying the molecules and inventing new devices to harness them. Such devices could harvest heat in, say, computers, to extend laptop battery life.