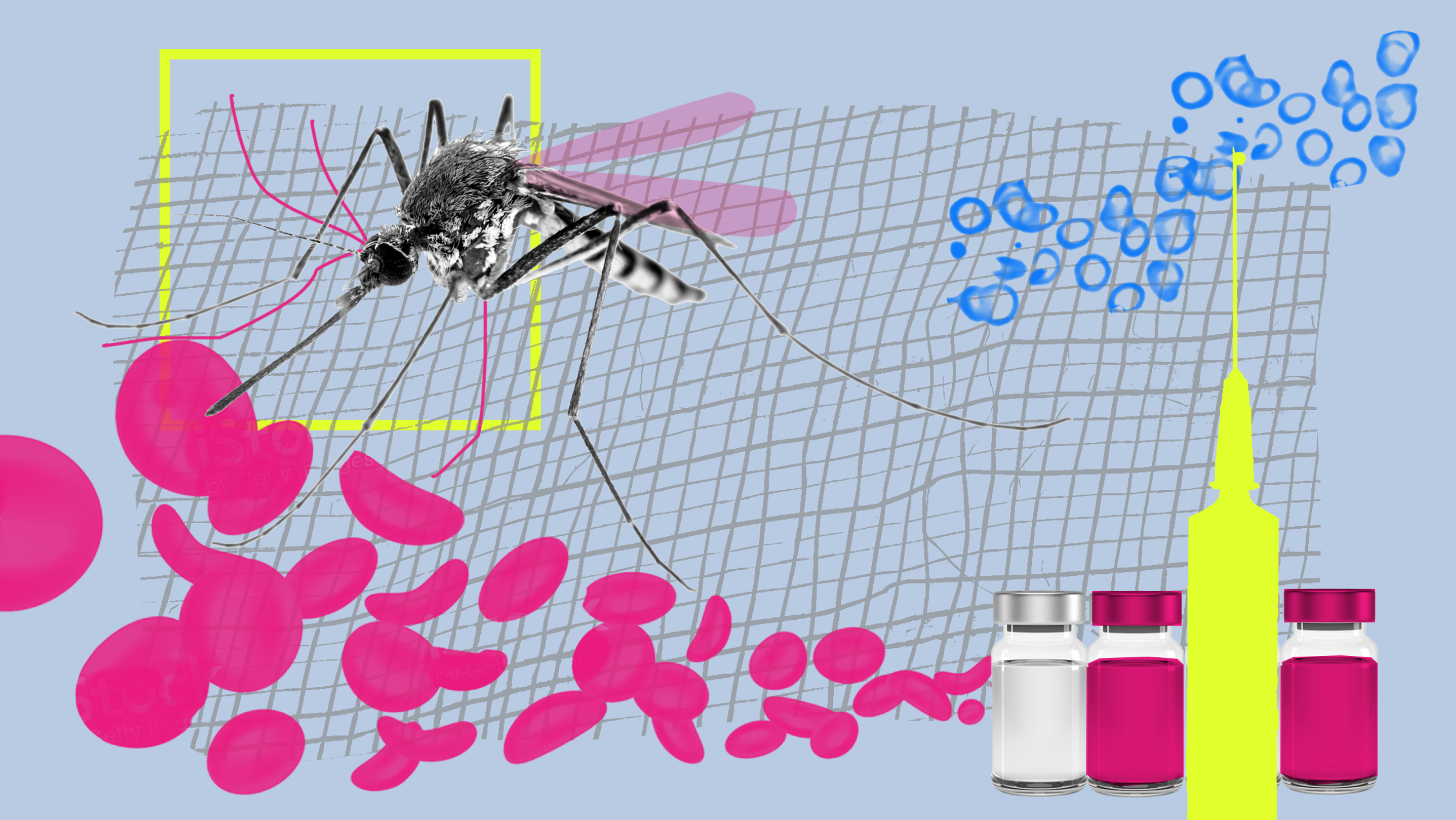
Malaria vaccine
Malaria kills hundreds of thousands of children a year. Combined with other measures, the vaccine could reduce deaths by as much as 70%.
Key players
GlaxoSmithKline, World Health Organization
Availability
Now (limited)
The malaria parasite, a notoriously deadly foe, has evolved countless ways to evade immune detection and thrive in human hosts. Mainly concentrated in sub-Saharan Africa, which accounts for roughly 95% of cases, malaria kills more than 600,000 people a year, a majority of them children younger than five years old.
Last October, after years of development, the World Health Organization finally approved the world’s first vaccine to combat the deadly mosquito-borne disease.
GlaxoSmithKline’s vaccine, known as RTS,S or Mosquirix, is not a particularly effective one. It requires three doses in children between five months and 17 months old, and a fourth dose given 12 to 15 months after that. Given to more than 800,000 children in Kenya, Malawi, and Ghana, the vaccine had an efficacy of about 50% against severe malaria in the first year, and its effectiveness dropped dramatically over time.
Even so, public health officials are hailing the vaccine, which has been in testing since 1987, as a “game changer” in Africa. When combined with other malaria control measures, which include insecticide-treated bed nets and preventative drugs administered during the rainy season, it is expected to reduce malaria deaths by as much 70%, compared with the death rate in children given existing drugs.
Mosquirix also has a broader significance: it is the first vaccine approved for a parasitic disease. Parasites are complex multicellular organisms, with genomes 500 to 1,000 times larger than those found in most viruses and bacteria. This complexity allows them to mutate in myriad ways when challenged by the immune response. Glaxo’s vaccine consists of copies of a single protein that dots the surface of the parasite early in its life, paired with a series of molecules designed to set off alarm bells in the immune system and catalyze the production of antibodies that will protect potential hosts against the real thing.
Public health officials say the approval is likely to encourage innovation. Second-generation malaria vaccines—as well as vaccines for other parasitic diseases—are already in the pipeline.
As part of our 10 Breakthrough Technologies series, examine how the malaria vaccine came about and where we go from here.
Deep Dive
Biotechnology and health
How scientists traced a mysterious covid case back to six toilets
When wastewater surveillance turns into a hunt for a single infected individual, the ethics get tricky.
An AI-driven “factory of drugs” claims to have hit a big milestone
Insilico is part of a wave of companies betting on AI as the "next amazing revolution" in biology
The quest to legitimize longevity medicine
Longevity clinics offer a mix of services that largely cater to the wealthy. Now there’s a push to establish their work as a credible medical field.
There is a new most expensive drug in the world. Price tag: $4.25 million
But will the latest gene therapy suffer the curse of the costliest drug?
Stay connected
Get the latest updates from
MIT Technology Review
Discover special offers, top stories, upcoming events, and more.