Automating cell therapy
Multiply Labs’ robotic clusters could ease major bottlenecks in the production of drugs for intractable diseases.
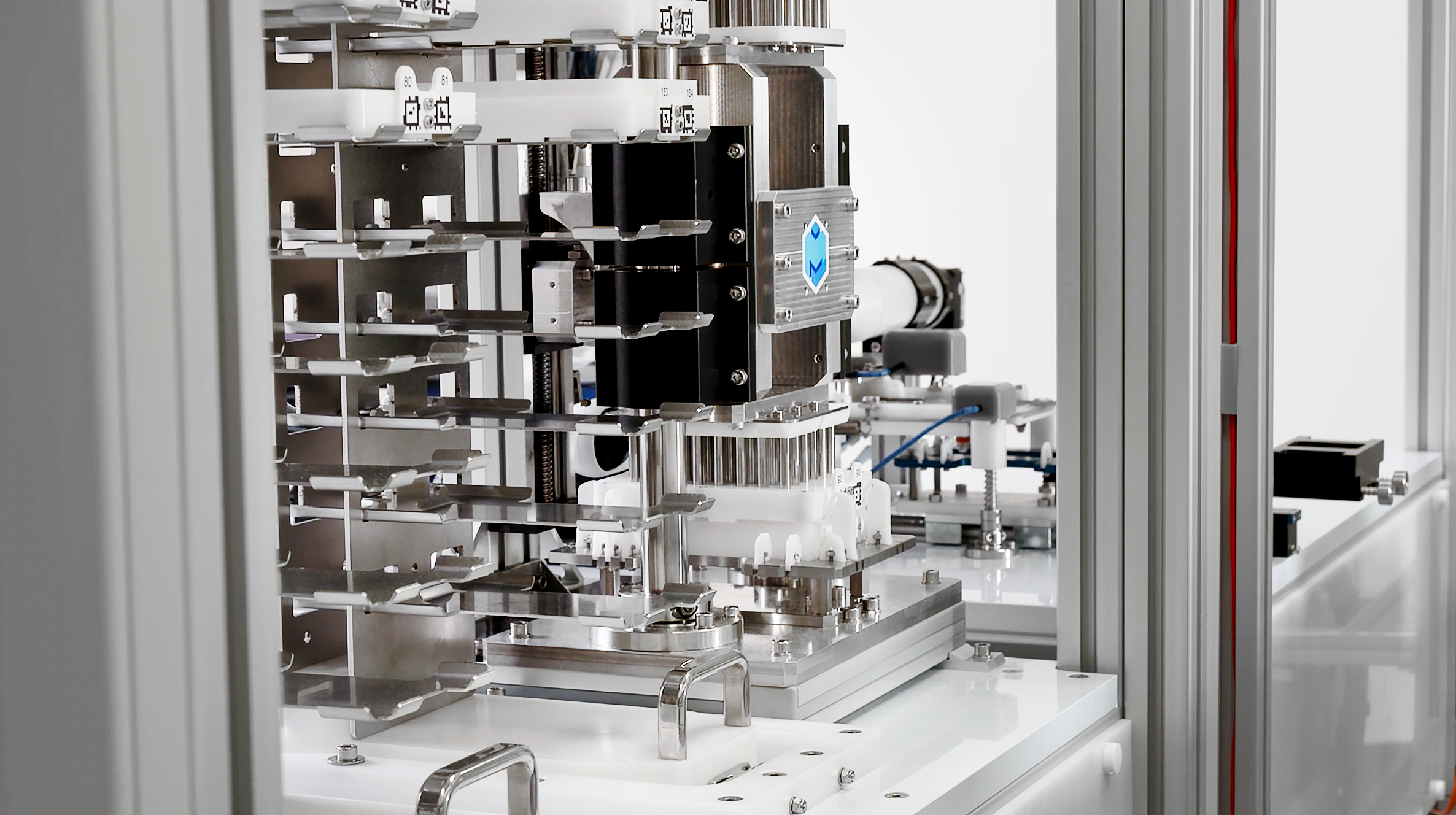
In the pristine production room of a pharmaceutical company, a robotic arm moves trays of drug capsules to and from stations in precise, efficient movements. On either side of the arm are 12-foot-tall stations made of modules stacked in twos. Each module contains equipment for one step in the process of making small batches of the drugs.
The machines, made by the robotics startup Multiply Labs, are automating the production of customized drug capsules for use in human clinical trials. And soon, if everything goes according to plan, these same robotic systems will be used to make advanced cell therapies.
Such therapies use cells, often ones involved in the immune system, as a way to treat patients with some of today’s most intractable diseases. A small handful of cell therapies have been approved by the US Food and Drug Administration. But making them is a complex, multistep process that is labor-intensive and often must be repeated for each individual patient to customize the treatment. There are more than 1,000 cell therapies in clinical trials today. If even a small fraction of these therapies are approved, the production bottleneck could be dire.
In other words, the process needs to be automated and speeded up. Multiply Labs’ robots are capable of working around the clock to produce 30,000 customized drug capsules per day. If they can bring that production power to cell therapies, it will transform the industry.
“We know there’s going to be a giant wave of cell therapies, and we know nobody knows how to make them [efficiently],” says Fred Parietti, PhD ’16, the company’s cofounder and CEO. “That’s why people are partnering with us.”
Getting personal
Parietti, who grew up in Italy, studied mechanical engineering as an undergraduate before coming to MIT in 2011 to pursue his PhD and work on robotics.
Cofounder Alice Melocchi, meanwhile, also grew up in Italy, but her interests were in chemical engineering. So Parietti was surprised to get a Facebook message in 2013 from Melocchi, at the time a visiting PhD candidate at MIT, saying she wanted a tour of the robotics lab Parietti was working in.
By that time, Melocchi had spent the past year meticulously creating drugs by hand in the Novartis-MIT Center for Continuous Manufacturing. When she toured Parietti’s lab, she saw a space littered not just with robotic controllers and limbs but also with long-forgotten robot parts from other projects. Parietti had taken to stacking the old robots on a big shelf in front of one of the windows.
What really caught Melocchi’s attention was the massive 3D printer the researchers used to make parts. Melocchi asked if the printer could be used to make personalized drugs—medicines whose ingredients are optimized for a specific individual.


Innovation at Multiply Labs is driven mostly by MIT researchers and engineers.
“I told her 3D printing is being used everywhere in general robotics, and I can’t believe nobody has used it for drug production,” Parietti recalls. “She said, ‘The industry is more focused on drug discovery, not drug manufacturing.’ I was like, ‘Oh, my God! Are you kidding me?’”
The founders’ original idea was to use 3D printing to make customized drug capsules. Ever the tinkerer, Parietti assembled a prototype of the robotic arm amid the pots and pans in his Cambridge kitchen. The MIT Sandbox Innovation Fund Program and Y Combinator, a startup accelerator, recognized the potential and funded and supported the team’s work. Parietti and Melocchi pinpointed their target market: pharmaceutical companies running clinical trials, which require drugs to be tested at different dosages.
“We know there’s going to be a giant wave of cell therapies, and we know nobody knows how to make them. That’s why people are partnering with us.”
Multiply Labs’ small team was steadfast in the belief that its work could improve drug production for clinical trials—even though, Parietti says, he and Melocchi met a lot of resistance and doubt.
“Potential advisors, potential customers, potential investors—‘This is impossible’ was the most common thing we heard,” he says. “It was funny, because first they would agree that personalized medicine was the future and that you cannot do it by hand, but then they’d say it’s impossible to solve it. I think it’s a very MIT thing to embrace big challenges, because people are used to being thrown hard problems.”
Today, those big challenges have been met. The company’s robotic clusters are used by leading pharma organizations. The cloud-based platforms are contained in 15 square feet and are completely modular, so companies can swap in parts and buy multiple complete systems to run together.
“To install a new part or system, we just connect it into the existing assembly,” Parietti says. “It’s pretty simple. It’s basically a Lego set.”
Multiply Labs expects great demand for its equipment. “We believe the future of medicine is going to be individualized drugs made on demand for single patients,” says Parietti, “and the only way to make those is with robots.”
Scaling cell therapy
Parietti’s team has spent the past three years learning about cell therapy production. Though these therapies are a promising tool against cancer, they are also expensive and time-consuming to manufacture. Take one FDA-approved approach, CAR-T therapy, which uses genetically engineered T cells. It requires scientists to extract blood from a patient, isolate immune cells, genetically modify those cells, grow more of the modified cells, and inject them back into the patient’s body. And given the nature of clinical trials, each step must be repeated for each patient.
The goal for Multiply Labs is to automate the steps now done by highly trained and skilled researchers, who spend endless hours completing the production tasks in serial processes.
The genetically modified cells are grown in bioreactors, which can take days. Parietti says Multiply Labs is planning to run a handful of bioreactor modules on its platform simultaneously, to ease the biggest bottleneck in the production process.
Multiply Labs’ researchers—almost all MIT engineers—realized early on that they approached problems very differently from people in pharmaceutical companies, hacking their way to working prototypes and then fine-tuning their systems to meet the standards of drug manufacturing.
“We’re all robotics people, so we already enjoy building robots, and since we’re building robots, we might as well build them to have the highest possible impact in the lives of people,” Parietti says. “I can’t think of a bigger impact than producing cell therapies, because these therapies are pretty staggering in terms of their effectiveness.”

Multiply Labs has assembled a consortium of key players to advance innovation in cell therapies. The global life sciences company Cytiva, which provides some of the most common bioreactors currently used for cell therapy production, is among the collaborators.
Parker Donner, Cytiva’s head of business development for cell and gene therapy, says that Multiply Labs’ automation technology “has broad implications for the industry that include expanding patient access to existing treatments and accelerating the next generation of treatments.”
Other partners include a manufacturing facility at the University of California, San Francisco, where Multiply Labs plans to demonstrate its first robotic system for cell therapies in March.
“It really is a great adventure for someone like me, a physician-scientist, to interact with mechanical engineers and see how they think and solve problems,” says Jonathan Esensten, an MD and assistant professor at UCSF whose research group is involved in the project.
Esensten says that last year his research group had a maximum capacity to produce no more than approximately two to three cell therapies per month, per manufacturing suite. “I’m hopeful we’ll build technologies that push this field forward and bend the cost curve to allow us to do things better, faster, and cheaper,” he says. “The beauty of the Multiply Labs concept is that it’s modular. You could imagine a robot where there are no bottlenecks: you have as much capacity as you need at every step, no matter how long it takes.”
“Cell therapies are amazing in terms of efficacy,” Parietti says. “But right now, they’re made by hand. Scientists are being used for manufacturing—it’s essentially artisanal. That’s not the way to scale.”
Keep Reading
Most Popular
Large language models can do jaw-dropping things. But nobody knows exactly why.
And that's a problem. Figuring it out is one of the biggest scientific puzzles of our time and a crucial step towards controlling more powerful future models.
The problem with plug-in hybrids? Their drivers.
Plug-in hybrids are often sold as a transition to EVs, but new data from Europe shows we’re still underestimating the emissions they produce.
How scientists traced a mysterious covid case back to six toilets
When wastewater surveillance turns into a hunt for a single infected individual, the ethics get tricky.
Google DeepMind’s new generative model makes Super Mario–like games from scratch
Genie learns how to control games by watching hours and hours of video. It could help train next-gen robots too.
Stay connected
Get the latest updates from
MIT Technology Review
Discover special offers, top stories, upcoming events, and more.