A gene-edited pig’s heart has been transplanted into a human for the first time
The procedure is a one-off, and highly experimental, but the technique could help reduce transplant waiting lists in the future.
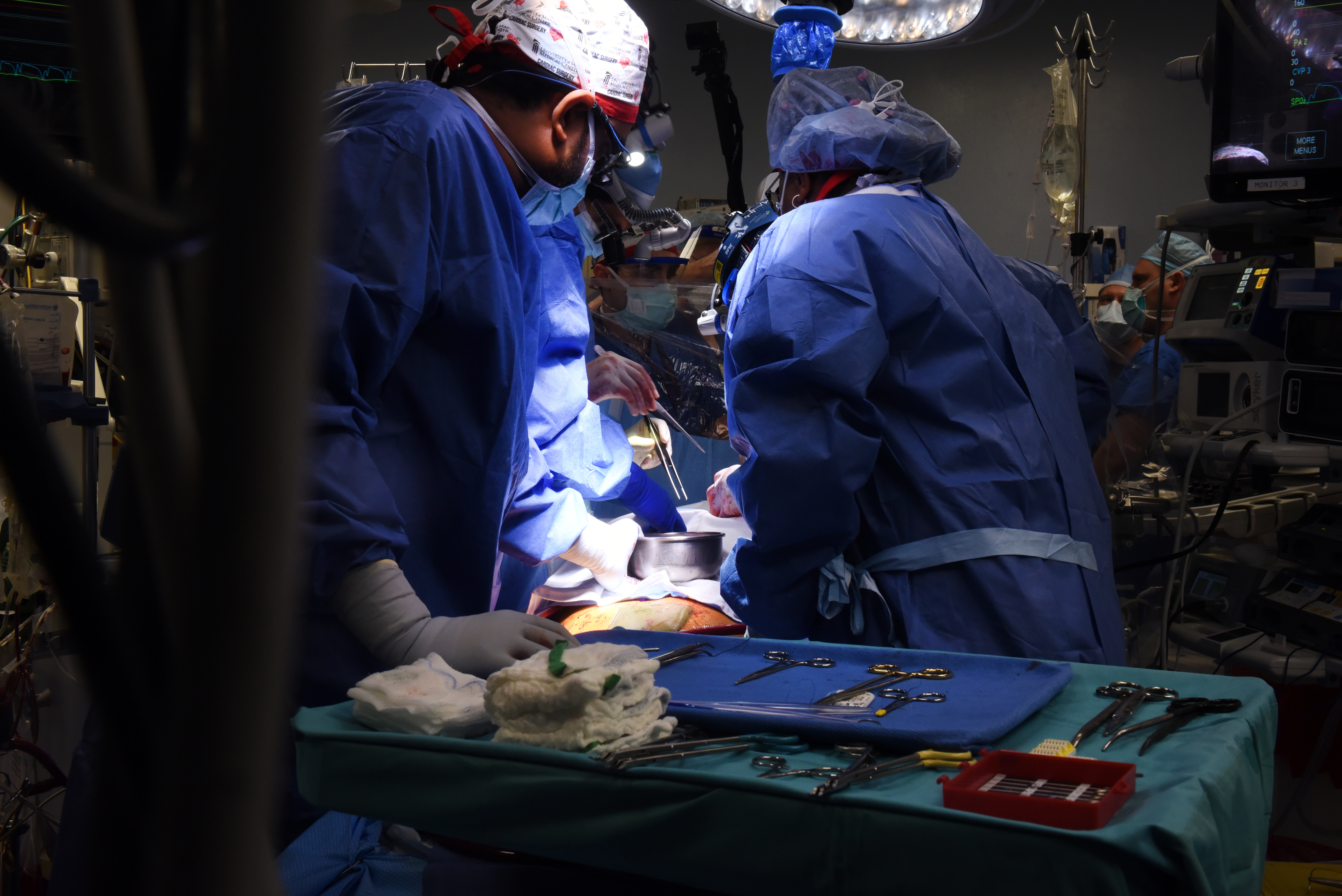
The news: A gene-edited pig’s heart has been transplanted into a human being for the first time. David Bennett Sr., a man with terminal heart disease, received the genetically modified heart during an eight-hour operation on Friday January 7 at the University of Maryland Medical Center, which issued a statement last night. The operation was a last-ditch effort on behalf of Bennett, 57, who had been deemed ineligible for a conventional heart transplant. He had been in the hospital for more than six weeks before the procedure with life-threatening arrhythmia. “It was either die or do this transplant,” he said in the press statement. “I want to live. I know it’s a shot in the dark, but it’s my last choice.”
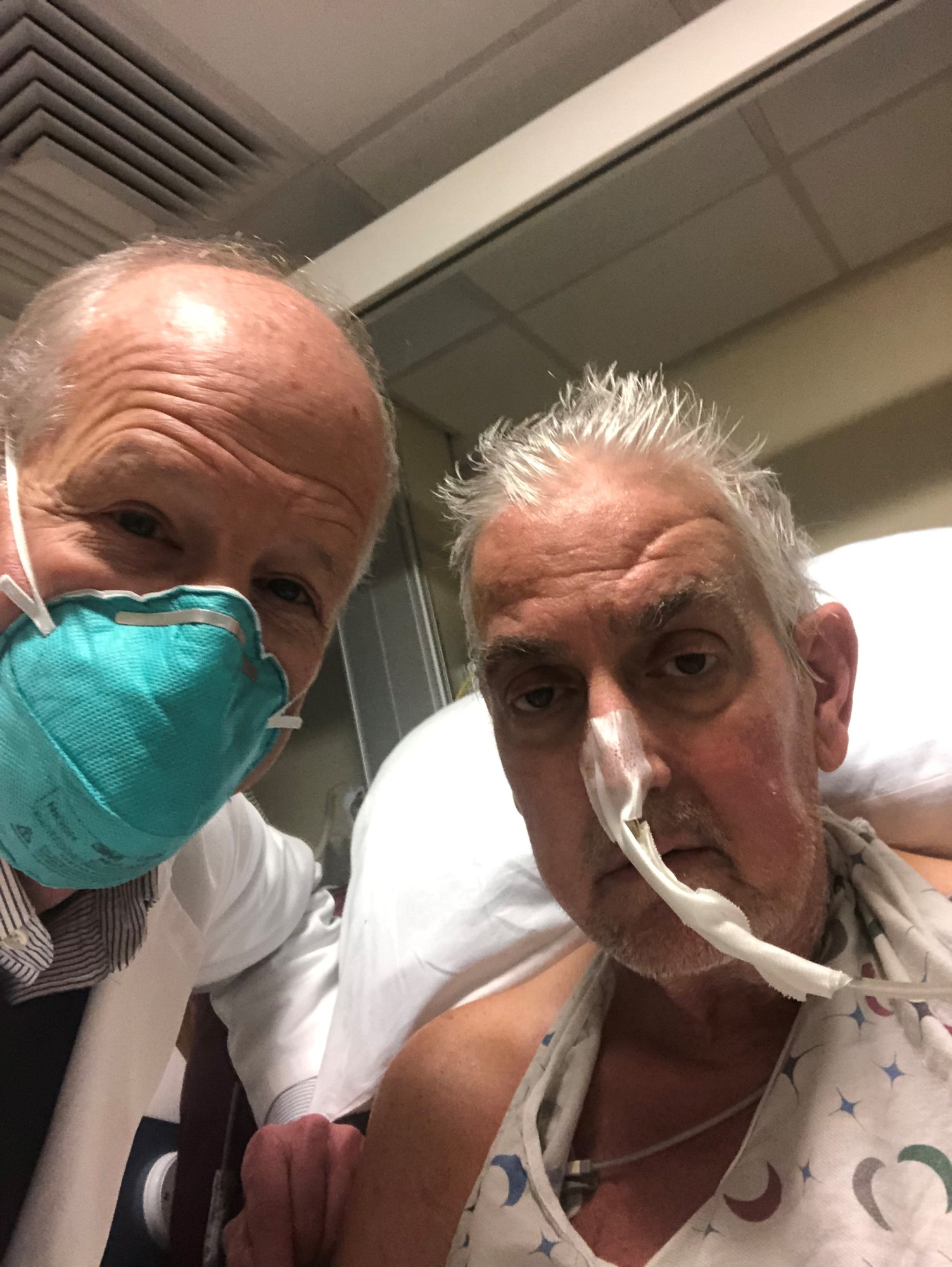
The technique: Ten genes in the donor pig were altered before the transplant could take place. Three of these genes cause human bodies to reject pig organs, so these were knocked out. Six genes were inserted to help control immune acceptance of the pig heart, and one additional gene was knocked out to stop excessive growth of the pig heart tissue.
The Maryland team also used an experimental drug to suppress the immune system and prevent rejection, and a new machine that pushed fluid through the tissue to make sure the pig’s heart remained viable until the procedure. The FDA gave emergency go-ahead for the procedure on New Year’s Eve, according to the New York Times.
What’s next: The demand for organs is vast, with nearly 107,000 people on the US transplant waiting list; 17 of them die every day, according to the Health Resources and Services Administration, a federal agency.
Early results look promising for Bennett, who is expected to come off the heart-lung bypass machine he’d been relying on to keep him alive today (January 11). He will be very closely monitored over the coming days and weeks for any signs of rejection or infection.
New frontier: While xenotransplantation, the process of transplanting animal organs or tissues into humans, has a long and often unsuccessful history, new gene-editing technologies are making it more viable. The gene-edited pig in last week’s operation was supplied by Revivicor, one of several biotech companies working to develop pig organs to transplant into humans.
Revivicor was also behind a successful transplantation of a pig’s kidney into a human patient last October, which was a major milestone in proving the viability of its techniques. As well as Revivicor, Harvard scientist George Church cofounded a company, eGensisis, that is working on using CRISPR gene editing to make animal organs viable for human transplant, although his ambitious proposed time scale has fallen off track.
Correction: We updated the first line to make it clear this was the first gene-edited heart.
Deep Dive
Biotechnology and health
How scientists traced a mysterious covid case back to six toilets
When wastewater surveillance turns into a hunt for a single infected individual, the ethics get tricky.
An AI-driven “factory of drugs” claims to have hit a big milestone
Insilico is part of a wave of companies betting on AI as the "next amazing revolution" in biology
The quest to legitimize longevity medicine
Longevity clinics offer a mix of services that largely cater to the wealthy. Now there’s a push to establish their work as a credible medical field.
There is a new most expensive drug in the world. Price tag: $4.25 million
But will the latest gene therapy suffer the curse of the costliest drug?
Stay connected
Get the latest updates from
MIT Technology Review
Discover special offers, top stories, upcoming events, and more.