Surgeons have successfully tested a pig’s kidney in a human patient
The test, in a brain-dead patient, was very short but represents a milestone in the long quest to use animal organs in human transplants.
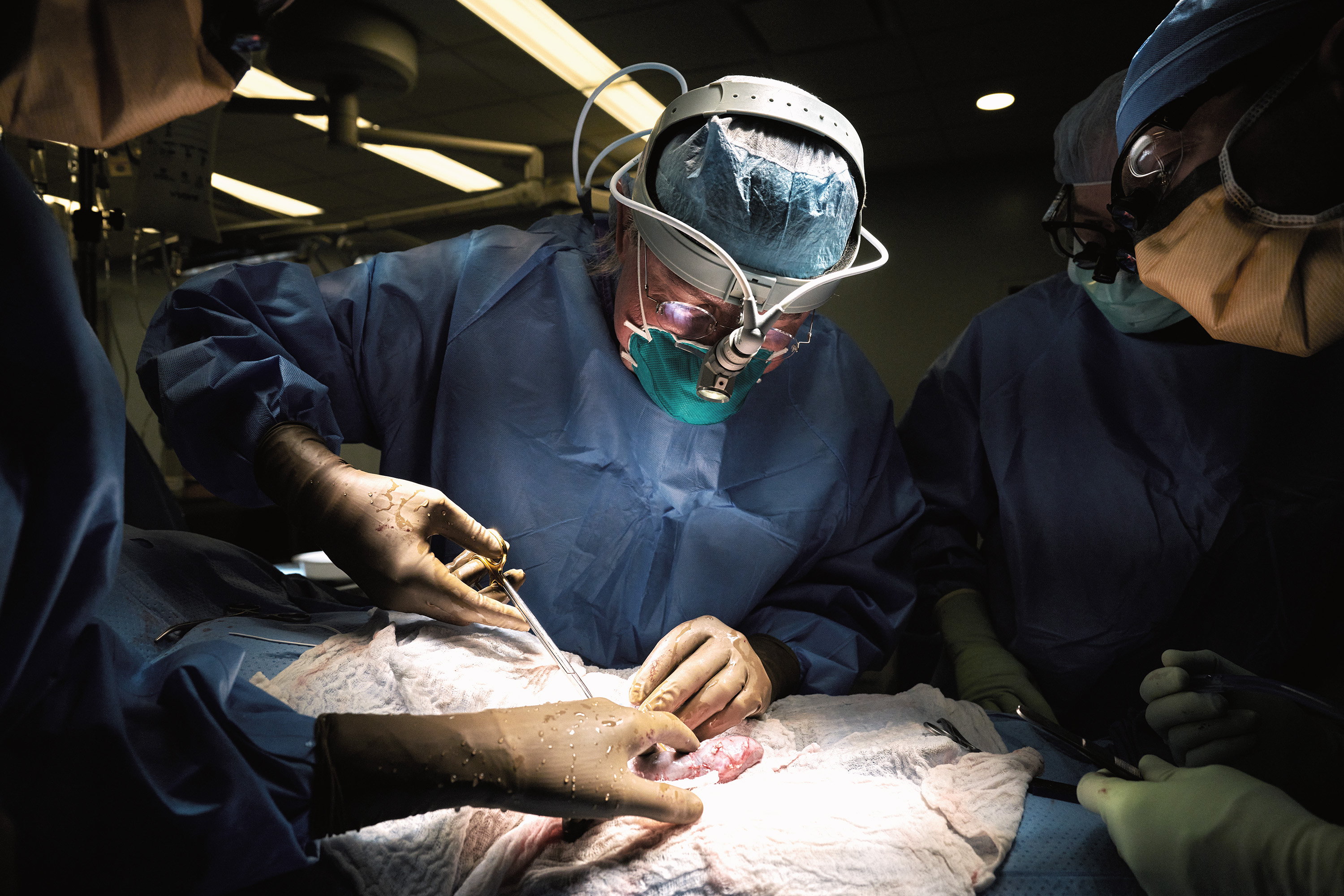
The news: Surgeons have successfully attached a pig’s kidney to a human patient and watched it start to work, the AP reported today. The pig had been genetically engineered so that its organ was less likely to be rejected. The feat is a potentially huge milestone in the quest to one day use animal organs for human transplants, which would shorten waiting lists.
How it worked: The surgical team, from NYU Langone Health, attached the pig kidney to blood vessels outside the body of a brain-dead woman and observed it for two days. The family agreed to the experiment before the woman was to be taken off life support, the AP reported. The kidney functioned normally—filtering waste and producing urine—and didn’t show signs of rejection during the short observation period.
The reception: The research was conducted last month and is yet to be peer reviewed or published in a journal, but external experts say it represents a major advance. “There is no doubt that this is a highly significant breakthrough,” says Darren K. Griffin, a professor of genetics at the University of Kent, UK. “The research team were cautious, using a patient who had suffered brain death, attaching the kidney to the outside of the body, and closely monitoring for only a limited amount of time. There is thus a long way to go and much to discover,” he added.
“This is a huge breakthrough. It’s a big, big deal,” Dorry Segev, a professor of transplant surgery at Johns Hopkins School of Medicine who was not involved in the research, told the New York Times. However, he added, “we need to know more about the longevity of the organ.”
The background: In recent years, research has increasingly zeroed in on pigs as the most promising avenue to help address the shortage of organs for transplant, but it has faced a number of obstacles, most prominently the fact that a sugar in pig cells triggers an aggressive rejection response in humans.
The researchers got around this by genetically altering the donor pig to knock out the gene encoding the sugar molecule that causes the rejection response. The pig was genetically engineered by Revivicor, one of several biotech companies working to develop pig organs to transplant into humans.
The big prize: There is a dire need for more kidneys. More than 100,000 people in the US are currently waiting for a kidney transplant, and 13 die of them every day, according to the National Kidney Foundation. Genetically engineered pigs could offer a crucial lifeline for these people, if the approach tested at NYU Langone can work for much longer periods.
Deep Dive
Biotechnology and health
How scientists traced a mysterious covid case back to six toilets
When wastewater surveillance turns into a hunt for a single infected individual, the ethics get tricky.
An AI-driven “factory of drugs” claims to have hit a big milestone
Insilico is part of a wave of companies betting on AI as the "next amazing revolution" in biology
The quest to legitimize longevity medicine
Longevity clinics offer a mix of services that largely cater to the wealthy. Now there’s a push to establish their work as a credible medical field.
There is a new most expensive drug in the world. Price tag: $4.25 million
But will the latest gene therapy suffer the curse of the costliest drug?
Stay connected
Get the latest updates from
MIT Technology Review
Discover special offers, top stories, upcoming events, and more.