The pain switch
Professor Fan Wang’s research is zeroing in on brain circuits that control the perception of pain. Her work could one day make it possible to manage pain without opioids.

Fan Wang spends most of her workdays in the confines of a lab, overseeing experiments, reviewing reams of data, and managing a team of research scientists.
Her lab in MIT’s Building 46, which she moved into upon joining the faculty in January, is pristine and orderly, a sterile place ruled by rigor and numbers, where daily interactions are as likely to be with research mice as with people.
But Wang thinks more these days about the human dimensions of her work and its implications in the messy realms of emotion and hope. After she and her colleagues published a paper on pain suppression in Nature Neuroscience last year, a flood of people—strangers living with unexplained, unrelieved, and relentless pain—poured into her life. They sent emails. They wanted her help. They wanted reassurance it wasn’t all in their head.
“They said, ‘Dr. Wang, I’m willing to be your guinea pig,’” she recalls. “It just shows how desperate they are.”
Wang, an investigator at the McGovern Institute for Brain Research and a professor of brain and cognitive sciences, has spent much of her career researching sensory perception and how the brain interprets touch and pain. She and her team are now working to understand new pain suppression centers in the brain with the hope of finding relief that doesn’t require opioids.
If they succeed, their work could profoundly shift the treatment of pain and reshape the lives of countless people, potentially preventing the cascading effects of addiction that often accompany opioid use.
“I just want to find a way to relieve pain,” Wang says, modestly.
But the brain areas she’s focused on—the places where anesthesia, pain, and sleep are controlled—are incredibly complex, and the task in front of her is monumental.
A wild guess
A self-described “science nerd,” Wang grew up in Beijing, first coming to the US as a graduate student. “August 25, 1993, I arrived at JFK,” she recalls, with typical precision and a touch of nostalgia.
Attending Columbia University, living in a cockroach-infested apartment, and sitting in the nosebleed seats at the Met, Wang was besotted with New York City and all its opportunities. She was mentored at Columbia by Nobel laureate Richard Axel and did her postdoctoral research at Stanford University with Marc Tessier-Lavigne. In 2003, she landed at Duke University, where she rose through the ranks from assistant professor to full professor of neurobiology at the medical school before coming to MIT.
Wang has always been interested in sensory perception, and her early research focused on the sense of smell, tracing the sensory neurons from the nasal cavity to the olfactory bulb, the part of the brain responsible for processing the perception of odors. Researchers had only a limited understanding of pain perception, she says. That remains true decades later.
“We know that there are sensory endings in the skin and fingers that make you feel temperature and texture. This perception is created in the brain,” Wang says. “I wanted to understand that at higher levels—to understand the development of the system.”
Wang was inspired and intrigued by the work of the American physician Henry Beecher, who famously documented cases of World War II soldiers who suffered extreme injuries but felt no pain when they were taken from the battlefield to military hospitals. “They should be in pain, but they’re not. Their brain is switched to a state where they feel no pain,” Wang explains. Even though their sensory neurons remained intact, she says, these stimulus detectors didn’t register the perception of pain: “That’s in the brain.”
She was also fascinated by reports of patients under general anesthesia, undergoing surgeries, who were conscious—some even recalled hearing the surgeon speak—yet felt no pain.
What, she wondered, was the brain doing in these cases and how could that be harnessed to blunt pain?
In the 175 years since the first patient was successfully put under general anesthesia, researchers have not pinpointed exactly how it works. The prevailing theory has been that general anesthesia shuts the brain down, creating a loss of consciousness. But the examples of the soldiers and the patients who had awareness under anesthesia led Wang to wonder if a part of the anesthetized brain was still, in effect, working to suppress pain.
“There may be regions in the brain that are, paradoxically, activated by anesthetic,” Wang says. “And if you have an active mechanism, then you can flip it on like a switch and switch off pain. That was a wild guess.”
To look for such a region, Wang and her team anesthetized mice with four common anesthetics and then, using molecular markers, found clusters of neurons that those compounds activated in a part of the brain’s hypothalamus as well as in the amygdala. Since the hypothalamic neurons express a neuropeptide that was previously linked to reducing pain, Wang focused on studying them first. And she discovered to her surprise that they seemed to be linked not only to pain suppression but to the loss of consciousness experienced under general anesthesia. When she and her team activated the neurons using a technique she’d pioneered at Duke (called “capturing activated neural ensembles,” or CANE), the mice entered into a long, deep sleep. The finding that multiple anesthetics all fire up a region of the brain to promote a sleep-like state provided the first clear evidence that there are active mechanisms involved in anesthesia. Because chronic pain patients are known to have sleep problems, this region could be a potential target for future sleep aids, Wang says.
Building on that research, Wang and her colleagues turned their attention to the cluster of neurons they’d found in the amygdala that were also fired up by general anesthesia. Was it possible they might underlie its pain suppression function? It seemed unlikely, because the amygdala is the part of the brain most associated with fear and the human fight-or-flight response, triggered by the prospect of pain; it was not an area previously connected to anesthesia and active pain suppression.
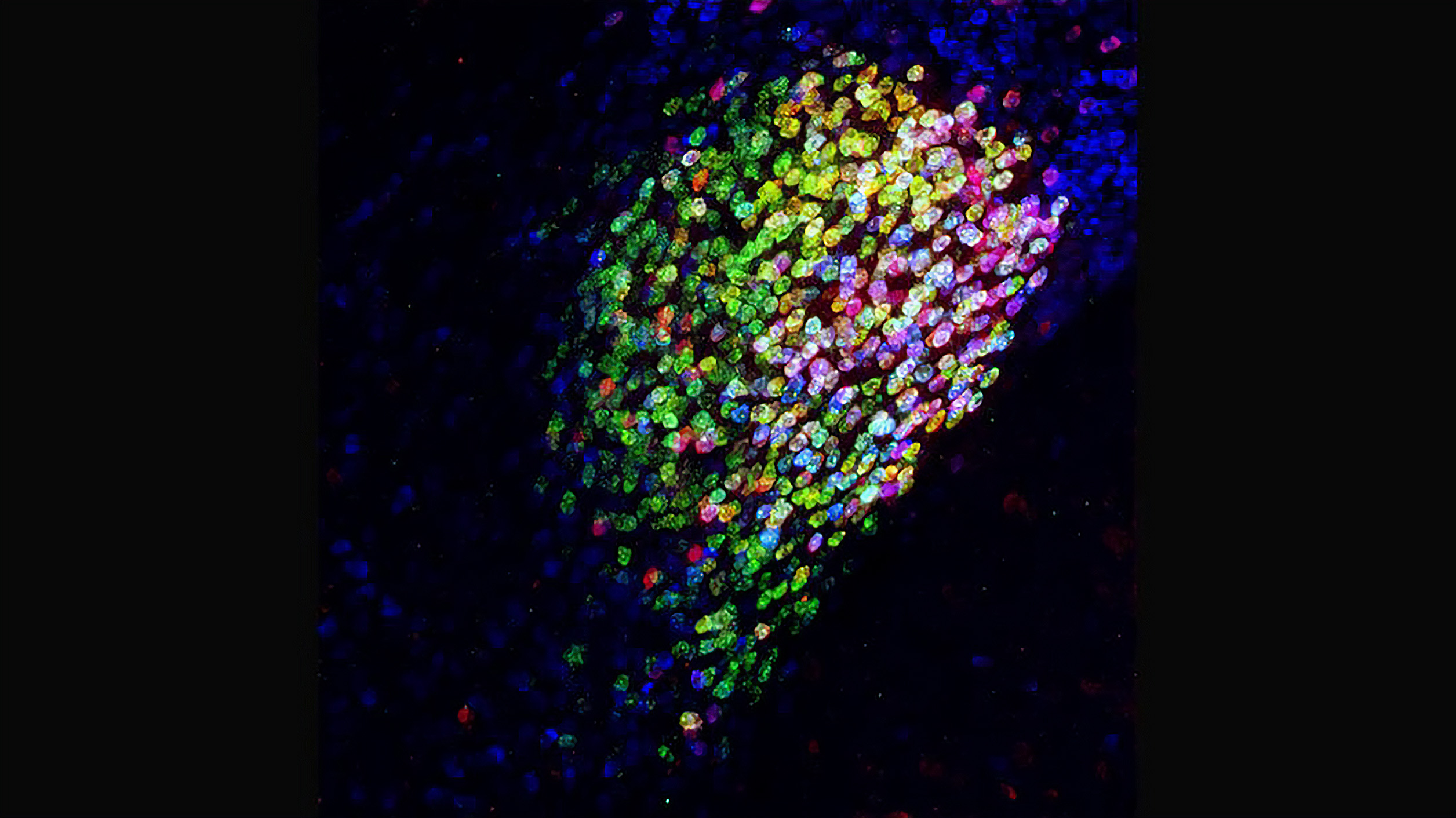
Remarkably, when Wang and her team used optogenetics to activate these specific central amygdala neurons, they discovered that the mice felt very little pain. Mice that had been exposed to an inflammatory agent or that had nerve pain caused by a chemotherapy drug or nerve pressure immediately stopped rubbing their faces and licking their paws—typical self-care behaviors induced by pain. Conversely, when the researchers deactivated these neurons, the mice responded to normal touch, such as stroking of the fur, as if it were painful. (Wang says that when her team imaged these neurons in their normal state, they found spontaneous ongoing activity, which they believe keeps the brain from being overly sensitive to normal touch.)
The researchers realized that these amygdala neurons were inhibitory cells, which slow or stop the activity of other neurons. And when they traced the neurons’ connections, they found that while they did not link to brain regions involved in sensing and discriminating between stimuli that might cause pain, they were linked to regions in the brain involved in processing the negative emotions and suffering associated with painful stimuli. In other words, it seemed that when the amygdala neurons were activated, the mice could sense stimuli that typically cause pain but did not experience the pain itself. The way these neurons were connected to many brain regions that process the negative emotions of pain suggested that the amygdala cells can inhibit all such regions.
This was a “holy grail” finding because it meant there was a single place in the brain that could, potentially, switch off pain. “We had this wild hypothesis and it turned out to be true,” Wang says, beaming.
An imprecise smoke alarm
When Nature Neuroscience published Wang’s research on the amygdala last year, media attention followed. And when people with chronic pain—an estimated 50 million Americans live with it—read that there was a possible “switch” deep within the brain that could relieve their unresolved suffering, they reached out to Wang’s lab.
Wang responded as delicately as she could. She told them that her research was only in mice at this stage, and that a human therapy was years off.
“I always have to apologize,” she says. “I say it’s only in rodents.”
When Wang discusses her research, she speaks quickly, moving her hands to accentuate her explanations, her brain seeming to race ahead. She feels an urgency about her work. But as excited and animated as she is about the possibilities, she also despairs. Recently she learned that one of the people who contacted her—a man with complex regional pain syndrome, causing pain that couldn’t be alleviated despite all his efforts—had taken his life.
“His whole body felt pain. Nothing relieved it,” Wang says, her voice cracking with emotion. “This is a terrible way of living a life. I can feel how terrible it is. And I’m so far away from turning my research into therapy.”
Another person recently asked her, “Why does God make us feel so much pain?” she recalls. “And it just made me so sad, because I didn’t have an answer.”
She does, however, have hope.
Her current research aims to pinpoint the neural circuit mechanisms that control how expectations and memories change our pain perception. She’s also exploring the contextual component of pain with the idea of treating it as “a perception problem.”
She is, she says, “very influenced by the rubber-hand illusion,” an experiment–slash–party trick in which people jump when a rubber hand, after being stroked the same way as their real hand, is suddenly struck with a hammer or knife . This, she thinks, suggests that the brain’s pain response in many cases may have very little to do with an actual painful stimulus.
“The system of pain is like an imprecise smoke alarm,” she explains. “Sometimes your brain interprets something as fire, but it’s really just a toasted piece of bread.”
“The system of pain is like an imprecise smoke alarm. Sometimes your brain interprets something as fire, but it’s really just a toasted piece of bread.”
So again working with mice, Wang and her team measured the animals’ responses to certain environments to test whether they could be trained to turn on the pain suppression switch themselves. They initially activated the central amygdala cells to give mice pain relief when they were in a box painted with shapes and stripes. In a box with different patterns—the control box—the mice did not receive pain relief. After a few training sessions, they tested the mice’s responses to various stimuli in the two boxes without activating the central amygdala cells. Interestingly, the animals showed much less pain response when they were placed in the “pain relief box” than when they were in the control box.
This showed, Wang says, that a context associated with pain relief could trigger the pain-suppressing switch in the amygdala. Eventually, humans might be conditioned in the same way, she says, without the need for drugs to activate the amygdala switch.
“Maybe you can train your brain to look at an app when the pain-suppressing amygdala cells are activated by drug or brain stimulation, so the brain remembers. Then later all you need to do is to look at the app again and the amygdala switches off the pain,” she says. “That’s the dream. That’s the future.”
In effect, the brain may ultimately be able to rejigger its perception of pain—to turn off pain that’s inexplicable and chronic or simply not useful.
“We haven’t had a new therapy for so long. Ibuprofen. Other drugs. They’re ancient,” Wang says. “We have to find another way.”
Wang is leading a new initiative studying addiction at the McGovern Institute, because she believes her work on pain suppression in the brain could eventually mean patients would no longer need opioids.
Her work with the addiction initiative is focusing on how drug use creates a “drug-craving” brain state that persists even after the physical dependence is treated and relieved. “I consider that as a deep mental pain,” she says.
The resources and collaborations at MIT make her feel that alleviating such pain is a real possibility. “This is the place,” she says. “I’m determined to find a way to relieve pain. These people are so desperate. They have so much faith in science, and I hope science doesn’t let them down.”
“Pain is very much a brain-generated illusion. All perceptions are illusions,” Wang says. “But just because it’s in the head doesn’t mean it’s not real. Everything’s in the head.”
The science of addiction
A new McGovern Institute initiative is tackling questions like why some people become addicted and others don’t.
About 130 people die every day in the US from opioid overdoses. Two of the top preventable causes of death are tobacco and alcohol. One in five children grows up in a home affected by addiction. Yet scientists still don’t understand the biology behind this chronic and complex brain disease.
A team of more than 20 McGovern Institute neuroscientists and engineers, led by Fan Wang, has begun work on a new effort to determine how addiction affects the brain and develop strategies to predict, prevent, and treat it.
More than 50% of patients relapse within six months of inpatient treatment for alcohol or drug addiction. Brain and cognitive sciences professor John Gabrieli’s lab is using brain imaging and machine learning to zero in on predictors of relapse and match patients with optimal interventions.
Polina Anikeeva, a professor of materials science and engineering, is developing tools that use light and magnetic fields to activate neurons in the brain’s reward region. By controlling these brain circuits in mice, she can study their role in drug-seeking behavior and look for ways to decrease responses linked to addiction. She’s also using fiber-based probes that she has created to pinpoint electrophysiological and neurochemical biomarkers of addiction.
Institute Professor and neuroscientist Ann Graybiel, PhD ’71, is exploring dopamine pathways linked to addiction and long-term habit formation. She’s also studying how addictive drugs hijack those pathways to drive motor behavior and habit formation. By looking at how these pathways differ in people with addictions, she hopes to shed light on why some people may be more prone to addiction than others.
Brain and cognitive sciences professor Ed Boyden ’99, MEng ’99, developed expansion microscopy technology that makes it possible to visualize microscopic connections between neurons and the locations of biomolecules within neurons. He’s now mapping molecular and neural circuit changes associated with addiction and will use machine learning to identify likely targets for treatment.
Biological engineering professor Alan Jasanoff, who developed MRI sensors that monitor neural activity, is now using new sensors that detect neurochemicals to study communication between brain regions linked to motivation, reward, and addiction. His goal is to better understand how addiction changes the way the brain works.
For more on the McGovern Institute’s addiction initiative, click here.
—Julie Pryor
Keep Reading
Most Popular
Large language models can do jaw-dropping things. But nobody knows exactly why.
And that's a problem. Figuring it out is one of the biggest scientific puzzles of our time and a crucial step towards controlling more powerful future models.
How scientists traced a mysterious covid case back to six toilets
When wastewater surveillance turns into a hunt for a single infected individual, the ethics get tricky.
The problem with plug-in hybrids? Their drivers.
Plug-in hybrids are often sold as a transition to EVs, but new data from Europe shows we’re still underestimating the emissions they produce.
Google DeepMind’s new generative model makes Super Mario–like games from scratch
Genie learns how to control games by watching hours and hours of video. It could help train next-gen robots too.
Stay connected
Get the latest updates from
MIT Technology Review
Discover special offers, top stories, upcoming events, and more.