Lab-grown mini-lungs could reveal why covid-19 kills
Inside the biosafety level 4 lab at the National Emerging Infectious Diseases Laboratories (NEIDL) in Boston, researchers wear three sets of gloves and breathe air piped into moon suits through snaking tubes. Before them, under a plastic shield, are human lung-sac cells grown from organoids, blobs of cells that mimic organs.
Now it’s time to infect them with the coronavirus.
What happens next could shed light on the strange and deadly effects of covid-19—because it’s not just the virus that matters, but the body’s reaction to it. People are dying from that reaction, and organoids could help zero in on where the damage is worst. Accurate cell models are already pinpointing how the virus gets into the body, where it causes the most harm, and will help in the search for treatments.
Many virologists work with computer data, or with surrogate viruses into which they plug parts of the covid-19 germ, or sometimes by infecting supplies of monkey cells that viruses like to grow in. But these surrogates can’t tell you what the actual virus does to specific human cell types. “If you work with the real thing, you get real results,” says Elke Mühlberger, a microbiologist at NEIDL, which is operated by Boston University. “If you are interested in the host response, then substitutes are of no use.”

One area where research on lab-made human lung tissue could pay off is in testing covid-19 drugs. Before trying any potential antiviral drug on people, researchers test their potency at blocking the virus in the lab. But after years of adaptation to a petri dish, standard laboratory cells are far from normal. “They’ve lost their ability to act as lung or liver, they don’t respond to interferon—they are very different than the real thing,” Mühlberger says. “They don’t do much other than get infected.”
Cells from organoids are different.
Tiny organs
Organoids are complex mini-tissues created from stem cells. These master cells are allowed to multiply and self-organize until they end up creating tiny clumps that can have the basic cellular makeup—and functions—of a real organ. There are mini-guts with delicate wrinkles, brain blobs that emit EEG waves, and structures that look surprisingly like real embryos.
Organoids had their debut as a virus-solver during the Zika outbreak, when infecting tiny lab brains showed that the virus had a preference for young, developing neurons. That offered an explanation for why the mosquito-borne germ was causing a birth defect, microcephaly, in some Brazilian newborns.
Organoids may also help researchers study animal viruses they haven’t had a good look at yet because they have proved difficult to grow in a laboratory setting. In May, scientists in Hong Kong cultivated mini-guts from horseshoe bats, the very species eyed as the root of the covid-19 outbreak, which harbor thousands of viruses about which we yet know little.
Lung cells
The research in Boston uses lung tissues being created at several area laboratories, including some that match parts of the alveoli, the puffy air sacs that exchange oxygen in the lung and that get overwhelmed in severe cases of covid-19.
Finn Hawkins, who runs one of the organoid labs, is a lung doctor who just finished a stint in an ICU caring for covid-19 patients. “I have never seen anything like it,” he says. “To me, the striking thing is the degree to which it causes severe lung damage in some patients. It’s not like Ebola, where everyone gets sick.”
The serious cases struggle with the same mysterious symptoms. Patients on ventilators are supposed to get weaned off. Instead, some are seized by a “cytokine storm,” an out-of-control inflammatory response, matched with a fever that won’t break. What’s killing most covid-19 patients is they come to the point where they can’t breathe at all. “Their markers go up; they need oxygen. The sudden worsening—that is something I have never seen before and now see over and over again,” says Hawkins. “You start to wonder what is going on, what’s driving the worsening.”
Hawkins says supplies of specific airway and lung cells could answer two questions: first, which cells let the virus into the body, and second, which are key to the devastating effects. Combine stem-cell-derived lung cells with the ability to sequence and track molecules inside individual cells and the result is “unbelievable resolution,” he says. “You can get information that is otherwise impossible to get.”
More on coronavirus
Our most essential coverage of covid-19 is free, including:
How does the coronavirus work?
What are the potential treatments?
What's the right way to do social distancing?
Other frequently asked questions about coronavirus
---
Newsletter: Coronavirus Tech Report
Zoom show: Radio Corona
See also:
Please click here to subscribe and support our non-profit journalism.
To see which cells are most susceptible, for instance, a team in North Carolina used sheets of human airway cells to form layers growing between liquid and air. They determined that ciliated cells—whose fuzzy hairs, waving like fronds on a sea anemone, move phlegm up and out—had the high levels of ACE-2, the human cell receptor the virus seizes on. Their findings pointed to the nose as the most likely place the virus gets in.
That’s information scientists may be able to use to come up with viral defenses. One video on YouTube suggested fashioning an anti-coronavirus nose closer out of a paper clip. If the virus is entering the nose, it’s not an entirely crazy idea. “If you can understand which types are getting infected and how, then you can find strategies to block or attenuate that,” says Hawkins.
The next question for the lung models will be to determine how particular cell types react to infection. “We suspect the virus is triggering something. What we are really interested in seeing is: when a virus gets in a cell, what are the downstream consequences?” says Hawkins. Some doctors think it’s damage to the lungs’ gas-exchange system, the alveoli, that leads to death. The air sac cells that exchange oxygen are big and thin, almost like the sails on a yacht. But the real problem could lie with “type 2” cells whose job is to make surfactant, a substance that reduces surface tension to let the air sacs stay open.
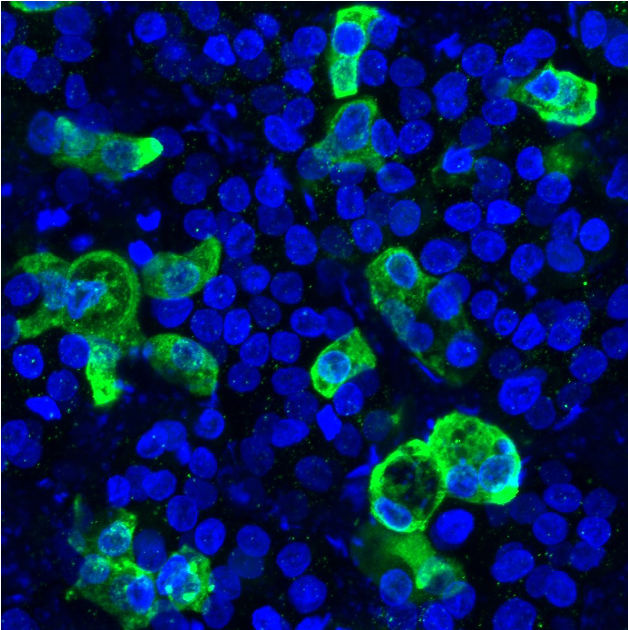
“If you look at the autopsies, the type 2 cells are very badly damaged. We know they are infected by the virus. That is the key cell type to understand what is killing these patients,” says Hawkins. “You get completely closed alveoli, and that’s what causes issues with oxygenation. When the patient ends up on the ventilators, it’s very challenging to deliver the right pressure and rescue these sick lungs that are leaky.”
Watching cells die
While Hawkins tends to patients on the wards, Jessie Huang, a postdoc at the Boston University lab of Darrell Kotton, has been manufacturing type 2 cells using organoids and sending them around the nation and the world. Alveolar cells from patients aren’t easy to grow, but the Boston labs have figured out how to generate them, and they can make a type of organoid called an alveolosphere.
Those cells are being transported to the secure labs across town. “Our part is super simple. We just add the virus,” says Mühlberger.
So what happens after giving lung cells covid-19? Mühlberger says she adds fluid with “a tiny amount of virus” to make the infection take hold. Within a few days the cells’ nuclei look fragmented, and some detach and float away. “You see the cells don’t do so well,” she says. “We think the virus kills the cells directly, but we don’t actually know.” It could be the overproduction of cytokines and chemokines, types of inflammation molecules.
Mühlberger believes organoids may also give a better idea of what drugs will work to block the virus from copying itself. One compound that stopped the virus in monkey cells didn’t help the lung cells at all, she says. She adds, “We think organoids have totally different response to virus, and the drugs might act differently too.”
Deep Dive
Biotechnology and health
How scientists traced a mysterious covid case back to six toilets
When wastewater surveillance turns into a hunt for a single infected individual, the ethics get tricky.
An AI-driven “factory of drugs” claims to have hit a big milestone
Insilico is part of a wave of companies betting on AI as the "next amazing revolution" in biology
The quest to legitimize longevity medicine
Longevity clinics offer a mix of services that largely cater to the wealthy. Now there’s a push to establish their work as a credible medical field.
There is a new most expensive drug in the world. Price tag: $4.25 million
But will the latest gene therapy suffer the curse of the costliest drug?
Stay connected
Get the latest updates from
MIT Technology Review
Discover special offers, top stories, upcoming events, and more.