Moderna’s latest vaccine results are promising—but it’s still too early
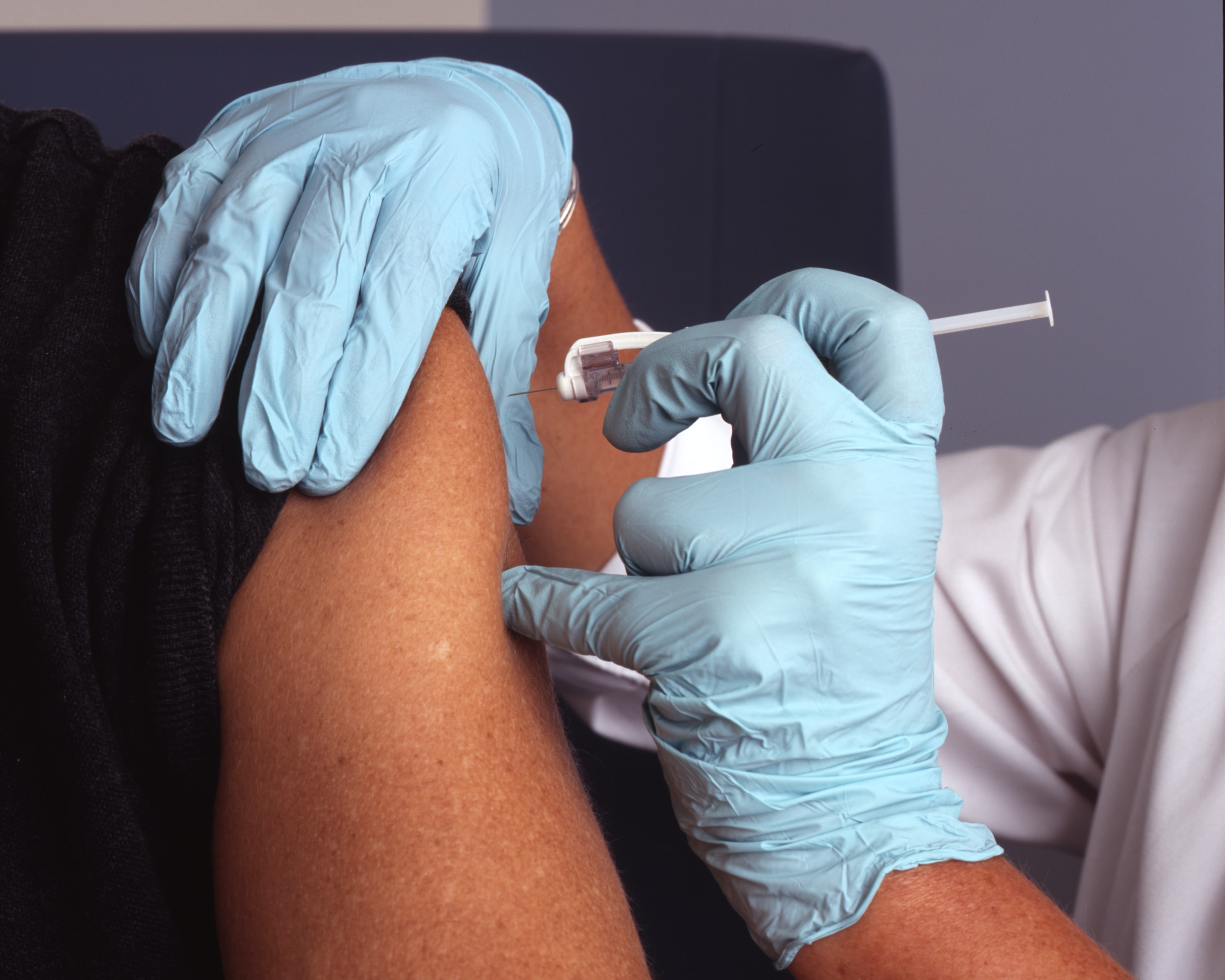
Drug maker Moderna has just announced encouraging interim results from the ongoing phase 1 trial for its experimental coronavirus vaccine. The early results suggest the vaccine has the potential to confer immunity against covid-19 in people.
What is the vaccine and how does it work? Moderna specializes in vaccines designed to elicit an immune response against the coronavirus through the virus’s mRNA—genetic instructions for the virus to replicate inside the host. New methods like Moderna’s are fast to prepare, but they have never led to a licensed vaccine for sale.
The phase 1 trial: The purpose of a phase 1 clinical trial is to establish whether or not the drug or vaccine being investigated is safe. It’s not designed to test for efficacy. That being said, the trials can still provide some insights into the potential of the drug or vaccine to treat patients. Moderna’s trial, which began in March and is still ongoing, is set to enroll up to 105 people. Each participant receives either two shots at 25 micrograms or 100 micrograms (about four weeks apart) or a single dose at 250 micrograms.
The new results: Moderna released antibody data for eight participants (aged 18 to 55), four in the 25-microgram arm and four in the 100-microgram arm. The results, according to the company, show that 25-microgram participants developed neutralizing antibodies to the coronavirus at levels comparable to recovered covid-19 patients; those in the 100-microgram arm had antibody levels that “significantly exceeded” the level in recovered patients. Adverse effects have been observed, but the company’s statement insists they “have been transient and self-resolving.”
Next steps: The results are encouraging, but we can’t draw conclusions from a phase 1 trial, with data from only eight participants so far. Way more data is needed. The FDA has already given Moderna approval for a phase 2 trial (testing the drug’s biological effect on patients), and Moderna says it will add a 50-microgram arm to the phase 2 study. A phase 3 trial is expected to start in July. According to the Wall Street Journal, if all Moderna’s trials go well—and that’s a big if—the FDA could grant emergency-use authorization for the vaccine by the fall.
Deep Dive
Biotechnology and health
How scientists traced a mysterious covid case back to six toilets
When wastewater surveillance turns into a hunt for a single infected individual, the ethics get tricky.
An AI-driven “factory of drugs” claims to have hit a big milestone
Insilico is part of a wave of companies betting on AI as the "next amazing revolution" in biology
The quest to legitimize longevity medicine
Longevity clinics offer a mix of services that largely cater to the wealthy. Now there’s a push to establish their work as a credible medical field.
There is a new most expensive drug in the world. Price tag: $4.25 million
But will the latest gene therapy suffer the curse of the costliest drug?
Stay connected
Get the latest updates from
MIT Technology Review
Discover special offers, top stories, upcoming events, and more.