U.S. Government Moves Forward with Tests of Novel Zika Vaccine

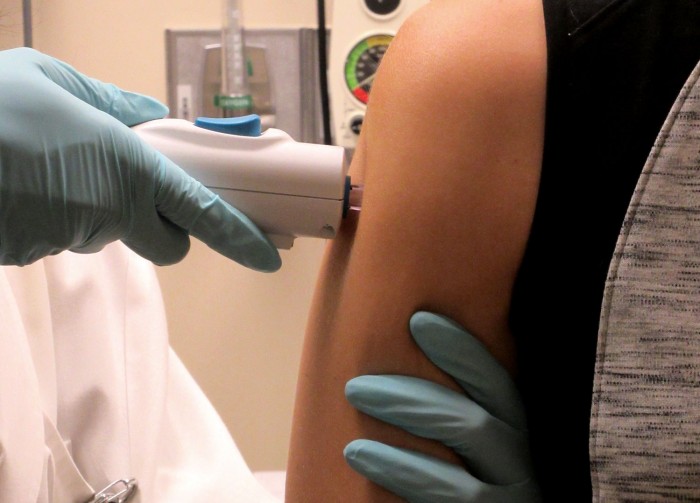
The U.S. government says it will move forward with wider tests of its Zika vaccine after the results from a safety study initiated last summer were promising.
The vaccine, called a DNA vaccine, uses genes from the virus to create an immune response. It was first given to volunteers last August as concern spread over a cluster of Zika cases in Miami.
The new phase of the vaccine tests began Wednesday at Baylor College of Medicine in Houston, one of 11 U.S. locations that will enroll up to 5,000 healthy adults, according to Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases, which developed the vaccine.
In a call with reporters on Friday, Fauci said initial results in 40 volunteers indicate that the vaccine produced an immune response against Zika and didn’t cause serious side effects.
Vaccine Push
Companies are developing novel DNA vaccines to protect against a range of infections and to treat cancer.
Astellas Pharma
Cytomegalovirus
GeneOne Life Science
Flu, hepatitis C
GeoVax Labs
HIV
Ichor Medial Systems
HIV, malaria, breast cancer, melanoma
Inovio Pharmaceuticals
MERS, Ebola, Zika
Madison Vaccines
Prostate cancer
Vical
Herpes
The threat has waned since Zika spread across Latin America and reached the U.S. last summer, but Fauci cautioned that the virus could return. People who get the mosquito-borne virus generally have only mild symptoms, but infection during pregnancy has been linked to serious birth defects.
The vaccine being tested, as well as other DNA vaccines, contains genetic material from the virus which, once injected into muscle, trains the body to mount an immune response against the virus.
The appeal of DNA vaccines is that they can be designed quickly to counter emerging threats and are easier to manufacture than traditional vaccines. The NIH previously developed a DNA vaccine against West Nile virus, but it was never commercialized.
Companies developing DNA vaccines include Astella Pharma, Inovio Pharmaceuticals, and GeoVax, whose products aim to protect against malaria, peanut allergy, and some types of cancer. So far, however, no DNA vaccine has reached the market.
To prove that the Zika vaccine protects against infection, Fauci says, the trial will eventually be expanded to sites in Puerto Rico, Brazil, Peru, Costa Rica, Panama, and Mexico, where the virus is still being transmitted.
Keep Reading
Most Popular
Large language models can do jaw-dropping things. But nobody knows exactly why.
And that's a problem. Figuring it out is one of the biggest scientific puzzles of our time and a crucial step towards controlling more powerful future models.
The problem with plug-in hybrids? Their drivers.
Plug-in hybrids are often sold as a transition to EVs, but new data from Europe shows we’re still underestimating the emissions they produce.
Google DeepMind’s new generative model makes Super Mario–like games from scratch
Genie learns how to control games by watching hours and hours of video. It could help train next-gen robots too.
How scientists traced a mysterious covid case back to six toilets
When wastewater surveillance turns into a hunt for a single infected individual, the ethics get tricky.
Stay connected
Get the latest updates from
MIT Technology Review
Discover special offers, top stories, upcoming events, and more.