How Carbon Dioxide from the Air Can Boost Batteries
There is little economic incentive to capture carbon dioxide from power-plant exhaust or suck it directly from the air, but researchers from George Washington University and Vanderbilt University have hit on a possible motivation: using the gas to make materials for high-performance batteries.
Not only is the new process more cost-effective than existing techniques for making carbon nanotubes at a large scale, they say, but it could be a weapon against climate change.
Capturing and sequestering carbon dioxide is expensive and unproven at the scale needed to significantly reduce emissions. The deployment of carbon capture and storage (CCS) technology is well behind schedule if it is to play the role that the governments behind the recent Paris climate agreement are betting it can play. In the absence of climate policies like a cap-and-trade system or carbon tax, the economics just don’t work.
That might change if the gas could be turned into a valuable product. Researchers have previously devised methods for using carbon dioxide to make liquid fuels like methanol, but those products have been relatively low-value commodities. Given the cost of today’s batteries, the new method makes a kilogram of carbon dioxide six times more valuable than one that is converted into methanol, says Stuart Licht, a professor of chemistry at George Washington.
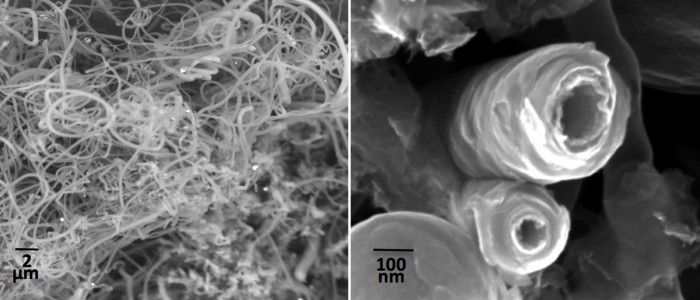
Replacing commonly used graphite anodes with carbon nanotubes can boost the storage capacity of advanced batteries. In proof-of-concept lab tests, Licht and his colleagues showed that nanotubes made with their process gave a small boost to the capacity of small lithium-ion batteries and almost quadrupled the capacity of sodium-ion batteries, an emerging energy storage technology.
The new result builds on a fundamental process Licht unveiled last year, which relies on molten lithium carbonate and lithium oxide. The lithium oxide, which is dissolved in the lithium carbonate, combines with carbon dioxide to make more lithium carbonate. When voltage is applied across two electrodes immersed in the molten lithium carbonate, an electrochemical reaction produces oxygen and pure carbon nanofibers. The power comes from a system Licht developed for concentrated solar energy, which makes use of infrared sunlight as well as visible light.
In this case the research group, led by Licht as well as Cary Pint, a professor of mechanical engineering at Vanderbilt, demonstrated a greater degree of control over the process. By adjusting various parameters, they produced carbon nanotubes that are “tailor-made” for anodes in lithium-ion and sodium-ion batteries, says Pint. In the lab tests the batteries containing the new anodes remained stable throughout many charging cycles.
Licht also proposed a design for fitting the system to a natural-gas power plant, where it would capture large amounts of carbon dioxide and convert it into carbon nanotubes and pure oxygen. The oxygen could then be used for further combustion, and the plant would have no carbon dioxide emissions, says Licht.
In the near future, says Licht, the group is looking forward to demonstrating the impressive strength of the carbon nanomaterials the process can make, which include solid carbon nanofibers in addition to the hollow tubes. “Eventually we are looking at perhaps making the casing of the battery from these materials also,” he says. “Or even better yet, the body of the car.”
Keep Reading
Most Popular
Large language models can do jaw-dropping things. But nobody knows exactly why.
And that's a problem. Figuring it out is one of the biggest scientific puzzles of our time and a crucial step towards controlling more powerful future models.
The problem with plug-in hybrids? Their drivers.
Plug-in hybrids are often sold as a transition to EVs, but new data from Europe shows we’re still underestimating the emissions they produce.
Google DeepMind’s new generative model makes Super Mario–like games from scratch
Genie learns how to control games by watching hours and hours of video. It could help train next-gen robots too.
How scientists traced a mysterious covid case back to six toilets
When wastewater surveillance turns into a hunt for a single infected individual, the ethics get tricky.
Stay connected
Get the latest updates from
MIT Technology Review
Discover special offers, top stories, upcoming events, and more.