Socks Generate Electricity Using Microbes Fed by Urine
For once, having socks full of urine and bacteria is a good thing. A pair of sock-like generators, designed by robotics professor Ioannis Ieropoulos’s team at the University of the West of England in Bristol, U.K., are able to turn human waste and locomotion into electrical power with a bit of help from microbes.
James Urquhart, reporting for New Scientist, has the details:
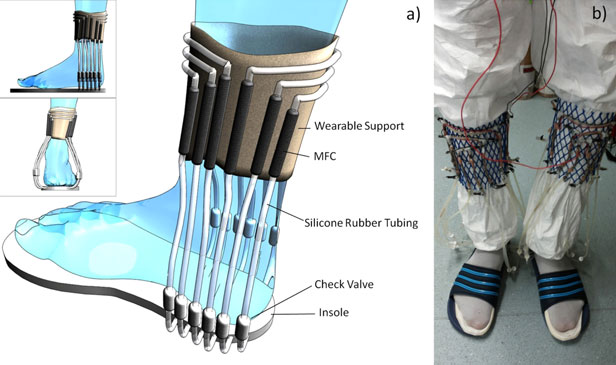
“Walking in the socks forces a bladder’s worth (roughly 648 millilitres) of urine to circulate through integrated tubes towards microbial fuels cells (MFCs), which contain bacteria that guzzle nutrients and create electricity.”
According to Ieropoulous’s team, this is the first time anyone has combined microbial fuel cells with wearable technology. Indeed, the socks produced enough electricity to power a specialized wireless transmitter sending out the message “World’s First Wearable MFC” every two minutes, reports Urquhart.
The crux of the idea was to create a self-contained system for generating power, with an eye toward survivalist scenarios. (Urquhart beat me to the Bear Grylls drink-my-own-urine joke.)
Using microbial fuel cells to generate electricity is nothing new; in fact it seems like Ieropoulos’s lab has cornered the market on piss-based power, operated “a mobile phone, a paper-based transmitter and a 3-D printed robotic heart” using what Utah Gary calls “liquid donations.” In these previous iterations, however, an external power source was needed to power pumps to move the urine around and keep the microbes fed.
The idea for the foot power was not, alas, inspired by Flintstones reruns but by the simplified circulatory system of fish, which is a single closed circuit powered by the simple pumping motion of the heart. Instead of muscular contractions, the sock uses the squeezing power of the human heel to drive the urine around so that it passes through 24 (24!) discrete, flexible MFCs positioned at different points around the sock.
Just as a fish’s muscles need their blood supply kept circulating to keep a fresh supply of oxygen available, the microbes in the MFCs need their urine bath constantly exchanged to ensure a steady supply of nutrients.
The prototype Ieropoulous and his team built did not yet address the issue how exactly to get “liquid donations” into the sock’s tubes. They seem confident, however, that such an obstacle could easily be overcome with a bit of smart textile design to channel the wearer’s waste into their device and exchange it once the nutrient supply ran out. (This calls to mind nothing so much as Dune’s stillsuits, devised to preserve every last drop of a person’s moisture on the desert planet Arrakis.)
In an ideal future, Ieropoulous sees the foot-pump system powering emergency devices to send out SOS signals for people caught away from traditional electricity sources and communications technology. Their system does not generate a tremendous amount of power, but in an outdoor emergency a single text message could make the difference between life and death.
Ieropoulous and his team published their findings in a highly readable paper in the journal Bioinspiration and Biomimetics.
Keep Reading
Most Popular
Large language models can do jaw-dropping things. But nobody knows exactly why.
And that's a problem. Figuring it out is one of the biggest scientific puzzles of our time and a crucial step towards controlling more powerful future models.
How scientists traced a mysterious covid case back to six toilets
When wastewater surveillance turns into a hunt for a single infected individual, the ethics get tricky.
The problem with plug-in hybrids? Their drivers.
Plug-in hybrids are often sold as a transition to EVs, but new data from Europe shows we’re still underestimating the emissions they produce.
Google DeepMind’s new generative model makes Super Mario–like games from scratch
Genie learns how to control games by watching hours and hours of video. It could help train next-gen robots too.
Stay connected
Get the latest updates from
MIT Technology Review
Discover special offers, top stories, upcoming events, and more.