Transplant Surgeons Revive Hearts After Death
Transplant surgeons have started using a device that allows them to “reanimate” hearts from people who have recently died, and use the organs to save others.
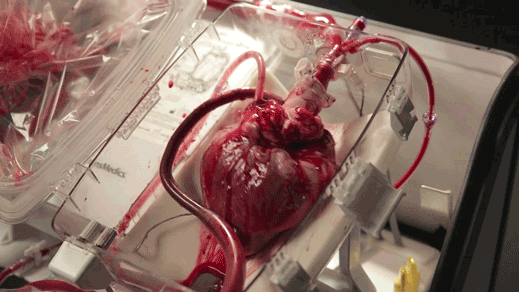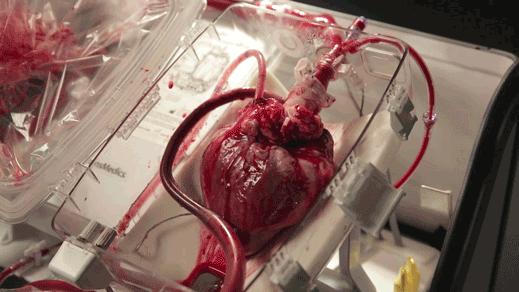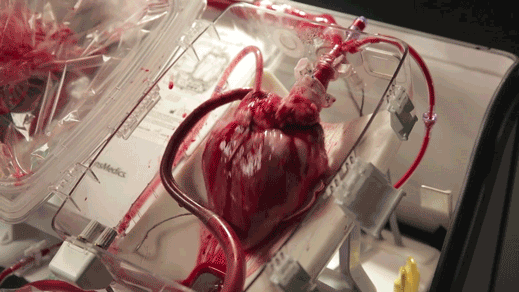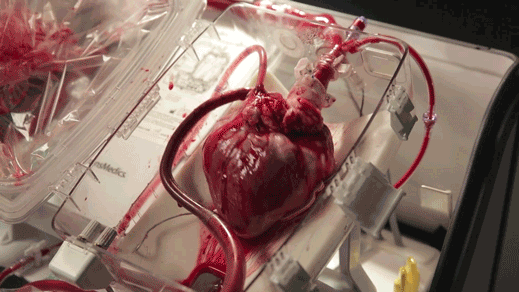
The “heart in a box” is a wheeled cart with an oxygen supply, a sterile chamber, and tubing to clamp onto a donor heart and keep it fed with blood and nutrients. Doctors say it may extend the time a heart can last outside the body and is letting them recover hearts from donors who haven’t been eligible before.
In at least 15 cases, surgeons in the United Kingdom and Australia say they have used the system to successfully transplant hearts removed from patients after they’ve died. Typically, heart transplants only come from brain-dead donors whose hearts are cut away while their bodies are still healthy.
The $250,000 device was developed by Transmedics, an Andover, Massachusetts-based company, and is pending approval in the U.S. It could expand the number of donated hearts by between 15 percent and 30 percent, say doctors, saving the lives of people who would otherwise die from heart failure.
In the U.S. about 2,400 heart transplant occur each year, a figure that has remained essentially unchanged for 20 years.
Earlier this year, in the Lancet, surgeons at St. Vincent’s Hospital in New South Wales described three cases in which they waited as little as two minutes after a person’s heart stopped before they began removing it. Within 20 minutes, they’d attached it to the Transmedics rig, where it began beating again after being fed with oxygenated blood and electrolytes.
Without such help, surgeons consider hearts from dead donors too damaged to use. “The device is vital. The heart gets an absolutely essential infusion of blood to restore its energy,” says Stephen Large, a surgeon at Papworth Hospital in the United Kingdom, which has used the system as part of eight heart transplants.
Transplant surgeons recognize two major categories of death. People can be brain dead, or they die because the heart and blood flow stop. The latter is what they now call “circulatory death.” But by the time it stops on its own, a person’s heart is starved of oxygen and the muscle cells are already dying. Left at body temperature, the damage, called ischemia, progresses rapidly.
That’s why heart surgeons have required hearts from brain-dead donors. These can be cooled down inside the body, then stopped, removed, and shipped at near 4 °C. Cold temperatures cut the tissue’s metabolic rate by about 90 percent, creating time to reach the recipient. Nearly all transplanted organs, including kidneys, are preserved this way.
The heart in a box is part of a wider shift away from shipping organs cold to keeping them warm and functioning. In recent tests of such techniques, called warm perfusion, scientists have shown they can cut off a pig’s leg then replace it 12 hours later if it receives a supply of nutrients.
“Cold is the old thing, and warm is the new thing,” says Korkut Uygun, a transplant surgeon at the Massachusetts General Hospital. “Warm is the way to go with metabolically active tissue.”
Several small companies are working on warm perfusion machines, including Organ Assist, based in the Netherlands, OrganOx of Oxford, U.K., as well as Organ Solution, a startup founded by Uygun to rescue livers from dead donors. Uygun believes the Transmedics machine is still too expensive and not yet automated enough. For instance, the amount of oxygen reaching the heart isn’t controlled automatically based on what the heart needs.
“In the short term they’ll open the field,” says Uygun. Eventually he thinks it might be possible to recover livers as much as an hour after death. Right now, most people on the liver transplant list die waiting. “Then the number of organs we’re talking about is huge.”
The first successful heart transplant, in 1967, was carried out in South Africa from a 25-year-old car accident victim whose heart had stopped. The organ was then moved a few feet to a second operating room. But surgeons found that hearts that stop naturally often didn’t start again, or can’t pump blood, so they came to rely entirely on brain-dead donors for organs.
The problem is that there are not nearly enough brain-dead donors, says Large, the Papworth surgeon. The crisis is particularly severe in the U.K., where handguns and some other firearms are prohibited, unlike in the U.S. There are more than twice as many heart donors per capita in the U.S. as in the U.K.
Large believes taking hearts from circulatory-death donors could expand the supply in the U.K. by almost a third, or an additional 50 hearts on top of the 180 a year available now. Others offer more conservative estimates. Such donors are already a source of about 15 percent of kidneys in some countries.
Donors at the Papworth hospital have included victims of car accidents and failed suicide attempts by hanging. They had severe brain damage but were not brain-dead. These patients are usually on mechanical ventilators and some, though not all, die shortly after their family chooses to remove life support.
If their hearts do stop, the ethical dilemma is how long surgeons should wait before swooping in to retrieve organs. In the U.S., the accepted standard is five minutes, although Colorado surgeons in 2008 took hearts from brain-damaged newborns after waiting only 75 seconds.
Robert Truog, a medical ethicist at Harvard University, says a question is whether these donors are really dead, given that their hearts can be restarted, including inside another person. “How can you say it’s irreversible, when the circulatory function is restored in a different body? We tend to overlook that because we want to transplant these organs,” says Truog. “My argument is that they are not dead, but also that it doesn’t matter” so long as they and family members have given consent. “They are dying and it’s permissible to use their organs. The question is whether they are being harmed, and I would say they are not.”
Large’s hospital, in a rural area a half hour drive from Cambridge, has taken some new and even more radical steps, he said in an interview.
In seven of the eight cases involving the Transmedics device, he says, his team restarted the heart inside the dead patient. Following cessation of circulation, his team has waited five minutes, then quickly clamped off the blood supply to the brain and restarted the donor’s heart without removing it.
This way the team effectively turns a circulatory death into a brain dead “beating heart” donor. With the heart pumping, Large says, it’s possible to check its condition with accuracy and also maintain the blood flow to the kidney and liver, preserving those organs as well. After being observed beating inside the donor, he says, the hearts were removed and placed on the Transmedics device for transport to the recipients. The team’s results are unpublished.
All eight transplants so far have been successful, he says. One patient was publicly identified as Huseyin Ulucan, a 60-year-old from London.
Deep Dive
Biotechnology and health
How scientists traced a mysterious covid case back to six toilets
When wastewater surveillance turns into a hunt for a single infected individual, the ethics get tricky.
An AI-driven “factory of drugs” claims to have hit a big milestone
Insilico is part of a wave of companies betting on AI as the "next amazing revolution" in biology
The quest to legitimize longevity medicine
Longevity clinics offer a mix of services that largely cater to the wealthy. Now there’s a push to establish their work as a credible medical field.
There is a new most expensive drug in the world. Price tag: $4.25 million
But will the latest gene therapy suffer the curse of the costliest drug?
Stay connected
Get the latest updates from
MIT Technology Review
Discover special offers, top stories, upcoming events, and more.