Injectable Implants Could Help Crack the Brain’s Codes
Understanding how the brain works—or doesn’t, as the case may be—depends on deciphering the patterns of electrical signals its neurons produce. Recording them requires inserting electrodes into the tissue. But the rigid devices traditionally used to record these signals, or to therapeutically stimulate certain regions, can damage the brain and elicit an immune response, and they tend not to work for very long.
Now researchers have shown that a new type of flexible electronic device, which can be delivered via injection, could be a gentler alternative.
In the near term, the technology could yield valuable insights about how the electrical activity of certain circuits, or networks of neurons, is related to discrete functions, like the creation of a lasting memory. It could also shed light on the brain’s dysfunctions, like schizophrenia or Parkinson’s disease (see “Cracking the Brain’s Codes” and “Shining a Light on Madness”). Further down the road, the concept could lead to a better way to deliver therapeutic stimulation to address neurodegenerative diseases, or a stable brain-computer interface that might help disabled people do things their condition usually wouldn’t allow them to do, like move prosthetic limbs or communicate (see “The Thought Experiment”).
One current therapeutic use of implanted electronics is called deep brain stimulation, which is FDA-approved and used to treat Parkinson’s disease. The therapy involves inserting electrodes into certain regions of the brain and producing electrical pulses meant to regulate abnormal ones. This approach is also being studied as a treatment for other disorders, such as epilepsy.
Today’s technology is limited, not by the electronics, which are already “really powerful,” but because the interface between the brain tissue and the electronics is far from ideal, says Charles Lieber, a professor of chemistry and chemical biology at Harvard University. Existing implantable electrodes are too large and rigid, and this “mechanical mismatch” leads to tissue damage and immune response, he says.
Over time the devices tend to lose their ability to record or stimulate the area of interest. Researchers can only record for a matter of days or weeks. Implants for deep brain stimulation often must be repositioned or have their settings adjusted, and usually don’t last for more than a few years.
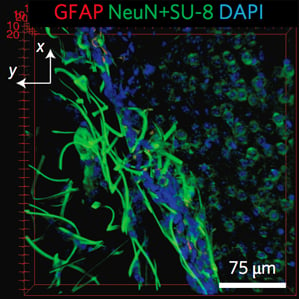
In recent years, a lot of progress has been made in research labs toward designing new kinds of implantable electronic devices made of more flexible and biocompatible materials (see “Wireless Micro LEDs Control Mouse Behavior”), but none have overcome this problem, Lieber says. The new nanoelectronic “mesh” structure that Lieber’s group has designed is much more like the biological tissue it is meant to interface with, he says. Its features are the size of cells or smaller, and, according to the researchers, the mesh is several orders of magnitude more flexible than any previous implantable electronic device.
The group originally conceived of the design a few years ago with tissue engineering in mind. They developed a scaffold-like structure that could support the growth of cells in three dimensions, similar to how it happens in the body, and also house electronic sensors for taking measurements from those cells. That demonstration suggested that the technology was potentially useful for measuring cellular activity.
Now they’ve shown that they can use a syringe to inject the mesh scaffold into targeted areas in the brains of live mice. They also demonstrated the ability to record signals from the injected implants by attaching a wire to a section of the mesh that remains outside the body. Tests indicate very little harmful tissue response, suggesting that the technology offers “substantial promise” for long-term brain activity mapping.
Made using conventional photolithography techniques, the mesh is composed of nanoscale metal wires and polymers. Tiny electronic devices, such as sensors and electrode stimulators, can be built into it. A scaffold that is a centimeter and a half in width can fold into a size small enough to be injectable through a needle whose diameter is just a few hundred micrometers. Once inside the body, it unfolds to conform to its 3-D environment. When it encounters a ventricular cavity in the brain, for example, it can unfold to fill in the space, bridging the two sides. Over time, neurons integrate with the mesh, providing the opportunity to record from or stimulate many single cells in a given region. The cells and the mesh have a “very positive interaction,” says Lieber, and the neurons “seem happy, at least over a month’s timescale.”
Lieber says his group has observed in unpublished experiments that it is possible to record for several months, from the same specific neurons, without signal degradation. He says the goal now is to demonstrate the same over six months to a year in mice before moving on to primates and, eventually, human trials.
Keep Reading
Most Popular
Large language models can do jaw-dropping things. But nobody knows exactly why.
And that's a problem. Figuring it out is one of the biggest scientific puzzles of our time and a crucial step towards controlling more powerful future models.
How scientists traced a mysterious covid case back to six toilets
When wastewater surveillance turns into a hunt for a single infected individual, the ethics get tricky.
The problem with plug-in hybrids? Their drivers.
Plug-in hybrids are often sold as a transition to EVs, but new data from Europe shows we’re still underestimating the emissions they produce.
Google DeepMind’s new generative model makes Super Mario–like games from scratch
Genie learns how to control games by watching hours and hours of video. It could help train next-gen robots too.
Stay connected
Get the latest updates from
MIT Technology Review
Discover special offers, top stories, upcoming events, and more.