How Nanomaterials Can Help Make Fuel from Sunlight
Researchers at the University of California, Berkeley, say that by combining nanoscale materials with bacteria, they have opened the door to a new way of designing systems that could efficiently turn carbon dioxide, water, and sunlight into useful organic compounds—similar to what plants do through photosynthesis. Down the road, they say, the system could become a commercially viable way to produce high-value chemicals like drug precursors used by the pharmaceutical industry, or to store renewable energy in the form of liquid fuels.
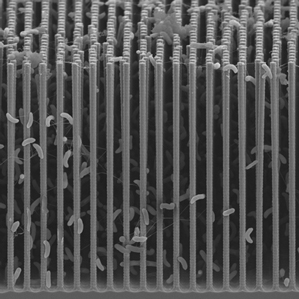
The goal of highly efficient artificial photosynthesis is a long-standing one, and there are many approaches to the problem, all of which face scientific hurdles (See “Sun + Water = Fuel” and “A Greener ‘Artificial Leaf’”). One general approach is to rely on microörganisms called electrotrophs, which can be coaxed, through the application of electricity, to make certain chemical building blocks.
The new system is the first one in which semiconductors, which are capable of both capturing solar energy and transmitting electricity to the microbes, have been directly combined with bacteria, says Peidong Yang, a professor of chemistry and materials science at the University of California, Berkeley, and an inventor of the system. Previous similar systems have relied on bulky solar panels to provide renewable electricity (see “Making Diesel from CO2 and Sunlight”). In this case, semiconducting nanowires capture energy from sunlight and pass electrons to electrotrophic bacteria, which are nestled within the wires. The electrotrophs use the electrons to turn carbon dioxide and water into useful chemical building blocks. Those are then passed to genetically engineered E. coli, which in turn make a wide range of products.
This is the first working example of such a direct interface between bacteria and semiconducting materials for artificial photosynthesis, says Yang. He and his colleagues demonstrated that the system could make butanol, a polymer used in biodegradable plastics, and three pharmaceutical precursors. It could in principle be used to make many other products, including chemicals that are valuable in relatively small volumes—unlike fuel, which must be produced at a very large scale to be economical.
The new system is about as efficient as natural photosynthesis at using the energy in sunlight, says Yang. That’s not enough for the process to be commercially viable, but he says new semiconductor materials his group is currently working with should make the process more competitive. “Efficiency is something we can improve in the near future,” he says.
An important potential advantage of this particular design, besides the light-capturing nanowire array, is that it can be used in the presence of oxygen, says Eric Toone, a professor of biochemistry and chemistry at Duke University and former director of ARPA-E’s electrofuels program, which focuses on developing technologies that use electrotrophic organisms to make fuel (the program funded Yang’s group while Toone was at the helm). The well-studied bacteria Yang’s group used cannot naturally tolerate oxygen, which has made the organism difficult to use at a large scale, says Toone. In the new design, says Yang, the nanowires “protect” the bacteria from oxygen.
Still, microbe-based systems face significant challenges because the bacteria must be kept alive, and even at best they don’t live very long. And compared with chemical catalysts, bacteria are slow “engines,” says Nate Lewis, a professor of chemistry at Caltech.
Indeed, Yang says his team’s ultimate goal is a synthetic system that is more stable than the bacteria-based system. But at the moment, he says, there are no better catalysts than bacteria for converting carbon dioxide into useful compounds. He and his colleagues are now looking closely at the way in which the semiconducting materials transfer electrons to the microbes. Investigating this semiconductor-bacteria interface could yield useful insights toward the design of a synthetic catalyst that could replace the bugs.
Keep Reading
Most Popular
Large language models can do jaw-dropping things. But nobody knows exactly why.
And that's a problem. Figuring it out is one of the biggest scientific puzzles of our time and a crucial step towards controlling more powerful future models.
How scientists traced a mysterious covid case back to six toilets
When wastewater surveillance turns into a hunt for a single infected individual, the ethics get tricky.
The problem with plug-in hybrids? Their drivers.
Plug-in hybrids are often sold as a transition to EVs, but new data from Europe shows we’re still underestimating the emissions they produce.
Google DeepMind’s new generative model makes Super Mario–like games from scratch
Genie learns how to control games by watching hours and hours of video. It could help train next-gen robots too.
Stay connected
Get the latest updates from
MIT Technology Review
Discover special offers, top stories, upcoming events, and more.