Engineering the Perfect Baby

If anyone had devised a way to create a genetically engineered baby, I figured George Church would know about it.
At his labyrinthine laboratory on the Harvard Medical School campus, you can find researchers giving E. Coli a novel genetic code never seen in nature. Around another bend, others are carrying out a plan to use DNA engineering to resurrect the woolly mammoth. His lab, Church likes to say, is the center of a new technological genesis—one in which man rebuilds creation to suit himself.
When I visited the lab last June, Church proposed that I speak to a young postdoctoral scientist named Luhan Yang. A Harvard recruit from Beijing, she’d been a key player in developing a powerful new technology for editing DNA, called CRISPR-Cas9. With Church, Yang had founded a small biotechnology company to engineer the genomes of pigs and cattle, sliding in beneficial genes and editing away bad ones.
As I listened to Yang, I waited for a chance to ask my real questions: Can any of this be done to human beings? Can we improve the human gene pool? The position of much of mainstream science has been that such meddling would be unsafe, irresponsible, and even impossible. But Yang didn’t hesitate. Yes, of course, she said. In fact, the Harvard laboratory had a project under way to determine how it could be achieved. She flipped open her laptop to a PowerPoint slide titled “Germline Editing Meeting.”
Here it was: a technical proposal to alter human heredity. “Germ line” is biologists’ jargon for the egg and sperm, which combine to form an embryo. By editing the DNA of these cells or the embryo itself, it could be possible to correct disease genes and pass those genetic fixes on to future generations. Such a technology could be used to rid families of scourges like cystic fibrosis. It might also be possible to install genes that offer lifelong protection against infection, Alzheimer’s, and, Yang told me, maybe the effects of aging. Such history-making medical advances could be as important to this century as vaccines were to the last.
That’s the promise. The fear is that germ-line engineering is a path toward a dystopia of superpeople and designer babies for those who can afford it. Want a child with blue eyes and blond hair? Why not design a highly intelligent group of people who could be tomorrow’s leaders and scientists?
Just three years after its initial development, CRISPR technology is already widely used by biologists as a kind of search-and-replace tool to alter DNA, even down to the level of a single letter. It’s so precise that it’s expected to turn into a promising new approach for gene therapy in people with devastating illnesses. The idea is that physicians could directly correct a faulty gene, say, in the blood cells of a patient with sickle-cell anemia (see “Genome Surgery”). But that kind of gene therapy wouldn’t affect germ cells, and the changes in the DNA wouldn’t get passed to future generations.
In contrast, the genetic changes created by germ-line engineering would be passed on, and that’s what has made the idea seem so objectionable. So far, caution and ethical concerns have had the upper hand. A dozen countries, not including the United States, have banned germ-line engineering, and scientific societies have unanimously concluded that it would be too risky to do. The European Union’s convention on human rights and biomedicine says tampering with the gene pool would be a crime against “human dignity” and human rights.
But all these declarations were made before it was actually feasible to precisely engineer the germ line. Now, with CRISPR, it is possible.
The experiment Yang described, though not simple, would go like this: The researchers hoped to obtain, from a hospital in New York, the ovaries of a woman undergoing surgery for ovarian cancer caused by a mutation in a gene called BRCA1. Working with another Harvard laboratory, that of antiaging specialist David Sinclair, they would extract immature egg cells that could be coaxed to grow and divide in the laboratory. Yang would use CRISPR in these cells to correct the DNA of the BRCA1 gene. They would try to create a viable egg without the genetic error that caused the woman’s cancer.
Yang would later tell me that she dropped out of the project not long after we spoke. Yet it remained difficult to know if the experiment she described was occurring, canceled, or awaiting publication. Sinclair said that a collaboration between the two labs was ongoing, but then, like several other scientists whom I’d asked about germ-line engineering, he stopped replying to my e-mails.
Regardless of the fate of that particular experiment, human germ-line engineering has become a burgeoning research concept. At least three other centers in the United States are working on it, as are scientists in China, in the U.K., and at a biotechnology company called OvaScience, based in Cambridge, Massachusetts, that boasts some of the world’s leading fertility doctors on its advisory board.
All this means that germ-line engineering is much further along than anyone imagined.
The objective of these groups is to demonstrate that it’s possible to produce children free of specific genes involved in inherited disease. If it’s possible to correct the DNA in a woman’s egg, or a man’s sperm, those cells could be used in an in vitro fertilization (IVF) clinic to produce an embryo and then a child. It might also be possible to directly edit the DNA of an early-stage IVF embryo using CRISPR. Several people interviewed by MIT Technology Review said that such experiments had already been carried out in China and that results describing edited embryos were pending publication. These people, including two high-ranking specialists, didn’t wish to comment publicly because the papers are under review.
All this means that germ-line engineering is much further along than anyone imagined. “What you are talking about is a major issue for all humanity,” says Merle Berger, one of the founders of Boston IVF, a network of fertility clinics that is among the largest in the world and helps more than a thousand women get pregnant each year. “It would be the biggest thing that ever happened in our field.” Berger predicts that repairing genes involved in serious inherited diseases will win wide public acceptance but says the idea of using the technology beyond that would cause a public uproar because “everyone would want the perfect child”: people might pick and choose eye color and eventually intelligence. “These are things we talk about all the time,” he says. “But we have never had the opportunity to do it.”
Editing embryos
How easy would it be to edit a human embryo using CRISPR? Very easy, experts say. “Any scientist with molecular biology skills and knowledge of how to work with [embryos] is going to be able to do this,” says Jennifer Doudna, a biologist at the University of California, Berkeley, who in 2012 co-discovered how to use CRISPR to edit genes.
To find out how it could be done, I visited the lab of Guoping Feng, a biologist at MIT’s McGovern Institute for Brain Research, where a colony of marmoset monkeys is being established with the aim of using CRISPR to create accurate models of human brain diseases. To create the models, Feng will edit the DNA of embryos and then transfer them into female marmosets to produce live monkeys. One gene Feng hopes to alter in the animals is SHANK3. The gene is involved in how neurons communicate; when it’s damaged in children, it is known to cause autism.
Feng said that before CRISPR, it was not possible to introduce precise changes into a primate’s DNA. With CRISPR, the technique should be relatively straightforward. The CRISPR system includes a gene-snipping enzyme and a guide molecule that can be programmed to target unique combinations of the DNA letters, A, G, C, and T; get these ingredients into a cell and they will cut and modify the genome at the targeted sites.
But CRISPR is not perfect—and it would be a very haphazard way to edit human embryos, as Feng’s efforts to create gene-edited marmosets show. To employ the CRISPR system in the monkeys, his students simply inject the chemicals into a fertilized egg, which is known as a zygote—the stage just before it starts dividing.
Feng said the efficiency with which CRISPR can delete or disable a gene in a zygote is about 40 percent, whereas making specific edits, or swapping DNA letters, works less frequently—more like 20 percent of the time. Like a person, a monkey has two copies of most genes, one from each parent. Sometimes both copies get edited, but sometimes just one does, or neither. Only about half the embryos will lead to live births, and of those that do, many could contain a mixture of cells with edited DNA and without. If you add up the odds, you find you’d need to edit 20 embryos to get a live monkey with the version you want.
That’s not an insurmountable problem for Feng, since the MIT breeding colony will give him access to many monkey eggs and he’ll be able to generate many embryos. However, it would present obvious problems in humans. Putting the ingredients of CRISPR into a human embryo would be scientifically trivial. But it wouldn’t be practical for much just yet. This is one reason that many scientists view such an experiment (whether or not it has really occurred in China) with scorn, seeing it more as a provocative bid to grab attention than as real science. Rudolf Jaenisch, an MIT biologist who works across the street from Feng and who in the 1970s created the first gene-modified mice, calls attempts to edit human embryos “totally premature.” He says he hopes these papers will be rejected and not published. “It’s just a sensational thing that will stir things up,” says Jaenisch. “We know it’s possible, but is it of practical use? I kind of doubt it.”
For his part, Feng told me he approves of the idea of germ-line engineering. Isn’t the goal of medicine to reduce suffering? Considering the state of the technology, however, he thinks actual gene-edited humans are “10 to 20 years away.” Among other problems, CRISPR can introduce off-target effects or change bits of the genome far from where scientists had intended. Any human embryo altered with CRISPR today would carry the risk that its genome had been changed in unexpected ways. But, Feng said, such problems may eventually be ironed out, and edited people will be born. “To me, it’s possible in the long run to dramatically improve health, lower costs. It’s a kind of prevention,” he said. “It’s hard to predict the future, but correcting disease risks is definitely a possibility and should be supported. I think it will be a reality.”
Editing eggs
Elsewhere in the Boston area, scientists are exploring a different approach to engineering the germ line, one that is technically more demanding but probably more powerful. This strategy combines CRISPR with unfolding discoveries related to stem cells. Scientists at several centers, including Church’s, think they will soon be able to use stem cells to produce eggs and sperm in the laboratory. Unlike embryos, stem cells can be grown and multiplied. Thus they could offer a vastly improved way to create edited offspring with CRISPR. The recipe goes like this: First, edit the genes of the stem cells. Second, turn them into an egg or sperm. Third, produce an offspring.
Some investors got an early view of the technique on December 17, at the Benjamin Hotel in Manhattan, during commercial presentations by OvaScience. The company, which was founded four years ago, aims to commercialize the scientific work of David Sinclair, who is based at Harvard, and Jonathan Tilly, an expert on egg stem cells and the chairman of the biology department at Northeastern University (see “10 Emerging Technologies: Egg Stem Cells,” May/June 2012). It made the presentations as part of a successful effort to raise $132 million in new capital during January.
During the meeting, Sinclair, a velvet-voiced Australian whom Time last year named one of the “100 Most Influential People in the World,” took the podium and provided Wall Street with a peek at what he called “truly world-changing” developments. People would look back at this moment in time and recognize it as a new chapter in “how humans control their bodies,” he said, because it would let parents determine “when and how they have children and how healthy those children are actually going to be.”
The company has not perfected its stem-cell technology—it has not reported that the eggs it grows in the lab are viable—but Sinclair predicted that functional eggs were “a when, and not an if.” Once the technology works, he said, infertile women will be able to produce hundreds of eggs, and maybe hundreds of embryos. Using DNA sequencing to analyze their genes, they could pick among them for the healthiest ones.
Genetically improved children may also be possible. Sinclair told the investors that he was trying to alter the DNA of these egg stem cells using gene editing, work he later told me he was doing with Church’s lab. “We think the new technologies with genome editing will allow it to be used on individuals who aren’t just interested in using IVF to have children but have healthier children as well, if there is a genetic disease in their family,” Sinclair told the investors. He gave the example of Huntington’s disease, caused by a gene that will trigger a fatal brain condition even in someone who inherits only one copy. Sinclair said gene editing could be used to remove the lethal gene defect from an egg cell. His goal, and that of OvaScience, is to “correct those mutations before we generate your child,” he said. “It’s still experimental, but there is no reason to expect it won’t be possible in coming years.”

Sinclair spoke to me briefly on the phone while he was navigating in a cab across a snowed-in Boston, but later he referred my questions to OvaScience. When I contacted OvaScience, Cara Mayfield, a spokeswoman, said its executives could not comment because of their travel schedules but confirmed that the company was working on treating inherited disorders with gene editing. What was surprising to me was that OvaScience’s research in “crossing the germ line,” as critics of human engineering sometimes put it, has generated scarcely any notice. In December of 2013, OvaScience even announced it was putting $1.5 million into a joint venture with a synthetic biology company called Intrexon, whose R&D objectives include gene-editing eggs to “prevent the propagation” of human disease “in future generations.”
When I reached Tilly at Northeastern, he laughed when I told him what I was calling about. “It’s going to be a hot-button issue,” he said. Tilly also said his lab was trying to edit egg stem cells with CRISPR “right now” to rid them of an inherited genetic disease that he didn’t want to name. Tilly emphasized that there are “two pieces of the puzzle”—one being stem cells and the other gene editing. The ability to create large numbers of egg stem cells is critical, because only with sizable quantities can genetic changes be stably introduced using CRISPR, characterized using DNA sequencing, and carefully studied to check for mistakes before producing an egg.
Tilly predicted that the whole end-to-end technology—cells to stem cells, stem cells to sperm or egg and then to offspring—would end up being worked out first in animals, such as cattle, either by his lab or by companies such as eGenesis, the spinoff from the Church lab working on livestock. But he isn’t sure what the next step should be with edited human eggs. You wouldn’t want to fertilize one “willy nilly,” he said. You’d be making a potential human being. And doing that would raise questions he’s not sure he can answer. He told me, “‘Can you do it?’ is one thing. If you can, then the most important questions come up. ‘Would you do it? Why would you want to do it? What is the purpose?’ As scientists we want to know if it’s feasible, but then we get into the bigger questions, and it’s not a science question—it’s a society question.”
Improving humans
If germ-line engineering becomes part of medical practice, it could lead to transformative changes in human well-being, with consequences to people’s life span, identity, and economic output. But it would create ethical dilemmas and social challenges. What if these improvements were available only to the richest societies, or the richest people? An in vitro fertility procedure costs about $20,000 in the United States. Add genetic testing and egg donation or a surrogate mother, and the price soars toward $100,000.
Others believe the idea is dubious because it’s not medically necessary. Hank Greely, a lawyer and ethicist at Stanford University, says proponents “can’t really say what it is good for.” The problem, says Greely, is that it’s already possible to test the DNA of IVF embryos and pick healthy ones, a process that adds about $4,000 to the cost of a fertility procedure. A man with Huntington’s, for instance, could have his sperm used to fertilize a dozen of his partner’s eggs. Half those embryos would not have the Huntington’s gene, and those could be used to begin a pregnancy.
Indeed, some people are adamant that germ-line engineering is being pushed ahead with “false arguments.” That is the view of Edward Lanphier, CEO of Sangamo Biosciences, a California biotechnology company that is using another gene-editing technique, called zinc fingers nucleases, to try to treat HIV in adults by altering their blood cells. “We’ve looked at [germ-line engineering] for a disease rationale, and there is none,” he says. “You can do it. But there really isn’t a medical reason. People say, well, we don’t want children born with this, or born with that—but it’s a completely false argument and a slippery slope toward much more unacceptable uses.”
Critics cite a host of fears. Children would be the subject of experiments. Parents would be influenced by genetic advertising from IVF clinics. Germ-line engineering would encourage the spread of allegedly superior traits. And it would affect people not yet born, without their being able to agree to it. The American Medical Association, for instance, holds that germ-line engineering shouldn’t be done “at this time” because it “affects the welfare of future generations” and could cause “unpredictable and irreversible results.” But like a lot of official statements that forbid changing the genome, the AMA’s, which was last updated in 1996, predates today’s technology. “A lot of people just agreed to these statements,” says Greely. “It wasn’t hard to renounce something that you couldn’t do.”
The fear? A dystopia of superpeople and designer babies for those who can afford it.
Others predict that hard-to-oppose medical uses will be identified. A couple with several genetic diseases at once might not be able to find a suitable embryo. Treating infertility is another possibility. Some men don’t produce any sperm, a condition called azoospermia. One cause is a genetic defect in which a region of about one million to six million DNA letters is missing from the Y chromosome. It might be possible to take a skin cell from such a man, turn it into a stem cell, repair the DNA, and then make sperm, says Werner Neuhausser, a young Austrian doctor who splits his time between the Boston IVF fertility-clinic network and Harvard’s Stem Cell Institute. “That will change medicine forever, right? You could cure infertility, that is for sure,” he says.
I spoke with Church several times by telephone over the last few months, and he told me what’s driving everything is the “incredible specificity” of CRISPR. Although not all the details have been worked out, he thinks the technology could replace DNA letters essentially without side effects. He says this is what makes it “tempting to use.” Church says his laboratory is focused mostly on experiments in engineering animals. He added that his lab would not make or edit human embryos, calling such a step “not our style.”
What is Church’s style is human enhancement. And he’s been making a broad case that CRISPR can do more than eliminate disease genes. It can lead to augmentation. At meetings, some involving groups of “transhumanists” interested in next steps for human evolution, Church likes to show a slide on which he lists naturally occurring variants of around 10 genes that, when people are born with them, confer extraordinary qualities or resistance to disease. One makes your bones so hard they’ll break a surgical drill. Another drastically cuts the risk of heart attacks. And a variant of the gene for the amyloid precursor protein, or APP, was found by Icelandic researchers to protect against Alzheimer’s. People with it never get dementia and remain sharp into old age.
Church thinks CRISPR could be used to provide people with favorable versions of genes, making DNA edits that would act as vaccines against some of the most common diseases we face today. Although he told me anything “edgy” should be done only to adults who can consent, it’s obvious to him that the earlier such interventions occur, the better.
Church tends to dodge questions about genetically modified babies. The idea of improving the human species has always had “enormously bad press,” he wrote in the introduction to Regenesis, his 2012 book on synthetic biology, whose cover was a painting by Eustache Le Sueur of a bearded God creating the world. But that’s ultimately what he’s suggesting: enhancements in the form of protective genes. “An argument will be made that the ultimate prevention is that the earlier you go, the better the prevention,” he told an audience at MIT’s Media Lab last spring. “I do think it’s the ultimate preventive, if we get to the point where it’s very inexpensive, extremely safe, and very predictable.” Church, who has a less cautious side, proceeded to tell the audience that he thought changing genes “is going to get to the point where it’s like you are doing the equivalent of cosmetic surgery.”
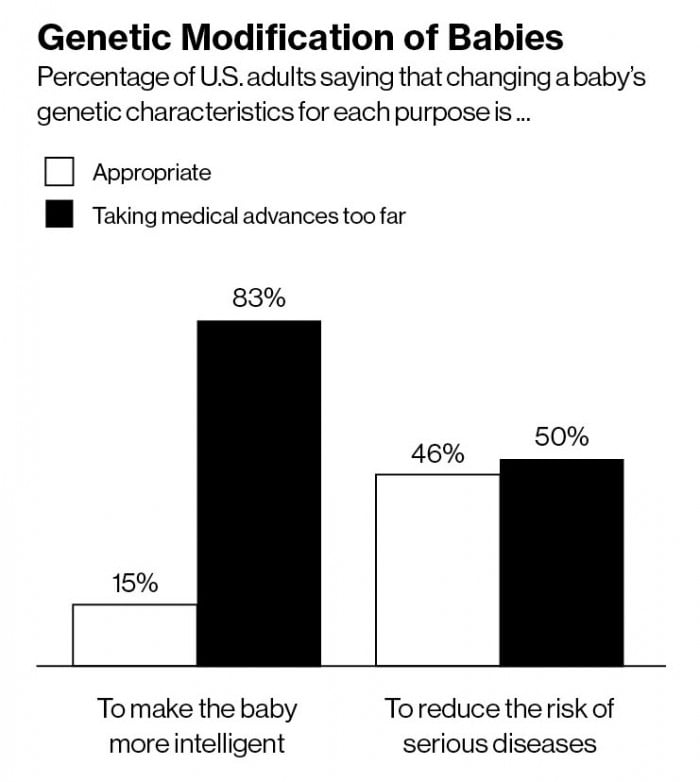
Some thinkers have concluded that we should not pass up the chance to make improvements to our species. “The human genome is not perfect,” says John Harris, a bioethicist at Manchester University, in the U.K. “It’s ethically imperative to positively support this technology.” By some measures, U.S. public opinion is not particularly negative toward the idea. A Pew Research survey carried out last August found that 46 percent of adults approved of genetic modification of babies to reduce the risk of serious diseases.
The same survey found that 83 percent said genetic modification to make a baby smarter would be “taking medical advances too far.” But other observers say higher IQ is exactly what we should be considering. Nick Bostrom, an Oxford philosopher best known for his 2014 book Superintelligence, which raised alarms about the risks of artificial intelligence in computers, has also looked at whether humans could use reproductive technology to improve human intellect. Although the ways in which genes affect intelligence aren’t well understood and there are far too many relevant genes to permit easy engineering, such realities don’t dim speculation on the possibility of high-tech eugenics.
“The human genome is not perfect. It’s ethically imperative to positively support this technology.”
What if everyone could be a little bit smarter? Or a few people could be a lot smarter? Even a small number of “super-enhanced” individuals, Bostrom wrote in a 2013 paper, could change the world through their creativity and discoveries, and through innovations that everyone else would use. In his view, genetic enhancement is an important long-range issue like climate change or financial planning by nations, “since human problem-solving ability is a factor in every challenge we face.”
To some scientists, the explosive advance of genetics and biotech means germ-line engineering is inevitable. Of course, safety questions would be paramount. Before there’s a genetically edited baby saying “Mama,” there would have to be tests in rats, rabbits, and probably monkeys, to make sure they are normal. But ultimately, if the benefits seem to outweigh the risks, medicine would take the chance. “It was the same with IVF when it first happened,” says Neuhausser. “We never really knew if that baby was going to be healthy at 40 or 50 years. But someone had to take the plunge.”
Wine country
In January, on Saturday the 24th, around 20 scientists, ethicists, and legal experts traveled to Napa Valley, California, for a retreat among the vineyards at the Carneros Inn. They had been convened by Doudna, the Berkeley scientist who co-discovered the CRISPR system a little over two years ago. She had become aware that scientists might be thinking of crossing the germ line, and she was concerned. Now she wanted to know: could they be stopped?
“We as scientists have come to appreciate that CRISPR is incredibly powerful. But that swings both ways. We need to make sure that it’s applied carefully,” Doudna told me. “The issue is especially human germ-line editing and the appreciation that this is now a capability in everyone’s hands.”
At the meeting, along with ethicists like Greely, was Paul Berg, a Stanford biochemist and Nobel Prize winner known for having organized the Asilomar Conference, a historic 1975 forum at which biologists reached an agreement on how to safely proceed with recombinant DNA, the newly discovered method of splicing DNA into bacteria.
Should there be an Asilomar for germ-line engineering? Doudna thinks so, but the prospects for consensus seem dim. Biotechnology research is now global, involving hundreds of thousands of people. There’s no single authority that speaks for science, and no easy way to put the genie back in the bottle. Doudna told me she hoped that if American scientists agreed to a moratorium on human germ-line engineering, it might influence researchers elsewhere in the world to cease their work.
Doudna said she felt that a self-imposed pause should apply not only to making gene-edited babies but also to using CRISPR to alter human embryos, eggs, or sperm—as researchers at Harvard, Northeastern, and OvaScience are doing. “I don’t feel that those experiments are appropriate to do right now in human cells that could turn into a person,” she told me. “I feel that the research that needs to be done right now is to understand safety, efficacy, and delivery. And I think those experiments can be done in nonhuman systems. I would like to see a lot more work done before it’s done for germ-line editing. I would favor a very cautious approach.”
Not everyone agrees that germ-line engineering is such a big worry, or that experiments should be padlocked. Greely notes that in the United States, there are piles of regulations to keep lab science from morphing into a genetically modified baby anytime soon. “I would not want to use safety as an excuse for a non-safety-based ban,” says Greely, who says he pushed back against talk of a moratorium. But he also says he agreed to sign Doudna’s letter, which now reflects the consensus of the group. “Although I don’t view this as a crisis moment, I think it’s probably about time for us to have this discussion,” he says.
(After this article was published online in March, Doudna’s editorial appeared in Science (see “Scientists Call for a Summit on Gene-Edited Babies”.) Along with Greely, Berg, and 15 others, she called for a global moratorium on any effort to use CRISPR to generate gene-edited children until researchers could determine “what clinical applications, if any, might in the future be deemed permissible.” The group, however, endorsed basic research, including applying CRISPR to embryos. The final list of signatories included Church, although he did not attend the Napa meeting.)
As news has spread of germ-line experiments, some biotechnology companies now working on CRISPR have realized that they will have to take a stand. Nessan Bermingham is CEO of Intellia Therapeutics, a Boston startup that raised $15 million last year to develop CRISPR into gene therapy treatments for adults or children. He says germ-line engineering “is not on our commercial radar,” and he suggests that his company could use its patents to prevent anyone from commercializing it.
“The technology is in its infancy,” he says. “It is not appropriate for people to even be contemplating germ-line applications.”
Bermingham told me he never imagined he’d have to be taking a position on genetically modified babies so soon. Modifying human heredity has always been a theoretical possibility. Suddenly it’s a real one. But wasn’t the point always to understand and control our own biology—to become masters over the processes that created us?
Doudna says she is also thinking about these issues. “It cuts to the core of who we are as people, and it makes you ask if humans should be exercising that kind of power,” she told me. “There are moral and ethical issues, but one of the profound questions is just the appreciation that if germ-line editing is conducted in humans, that is changing human evolution.” One reason she feels the research should slow down is to give scientists a chance to spend more time explaining what their next steps could be.
“Most of the public,” she says, “does not appreciate what is coming.”
This story was updated on April 23, 2015
Deep Dive
Biotechnology and health
How scientists traced a mysterious covid case back to six toilets
When wastewater surveillance turns into a hunt for a single infected individual, the ethics get tricky.
An AI-driven “factory of drugs” claims to have hit a big milestone
Insilico is part of a wave of companies betting on AI as the "next amazing revolution" in biology
The quest to legitimize longevity medicine
Longevity clinics offer a mix of services that largely cater to the wealthy. Now there’s a push to establish their work as a credible medical field.
There is a new most expensive drug in the world. Price tag: $4.25 million
But will the latest gene therapy suffer the curse of the costliest drug?
Stay connected
Get the latest updates from
MIT Technology Review
Discover special offers, top stories, upcoming events, and more.