Nanoparticle Disguised as a Blood Cell Fights Bacterial Infection
A nanoparticle wrapped in a red blood cell membrane can remove toxins from the body and could be used to fight bacterial infections, according to research published today in Nature Nanotechnology.
The results demonstrate that the nanoparticles could be used to neutralize toxins produced by many bacteria, including some that are antibiotic-resistant, and could counteract the toxicity of venom from a snake or scorpion attack, says Liangfang Zhang, a professor of nanoengineering at the University of California, San Diego. Zhang led the research.
The “nanosponges” work by targeting so-called pore-forming toxins, which kill cells by poking holes in them. One of the most common classes of protein toxins in nature, pore-forming toxins are secreted by many types of bacteria, including Staphylococcus aureus, of which antibiotic-resistant strains, called MRSA, are endemic in hospitals worldwide and cause tens of thousands of deaths annually. They are also present in many types of animal venom.
There are a range of existing therapies designed to target the molecular structure of pore-forming toxins and disable their cell-killing functions. But they must be customized for different diseases and conditions, and there are over 80 families of these harmful proteins, each with a different structure. Using the new nanosponge therapy, says Zhang, “we can neutralize every single one, regardless of their molecular structure.”
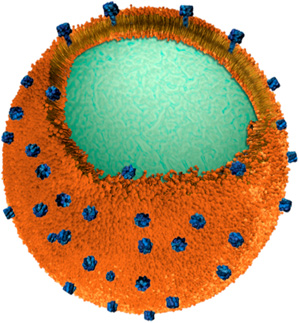
Zhang and his colleagues wrapped real red blood cell membranes around biocompatible polymeric nanoparticles. A single red blood cell supplies enough membrane material to produce over 3,000 nanosponges, each around 85 nanometers (a nanometer is a billionth of a meter) in diameter. Since red blood cells are a primary target of pore-forming toxins, the nanosponges act as decoys once in the bloodstream, absorbing the damaging proteins and neutralizing their toxicity. And because they are so small, the nanosponges will vastly outnumber the real red blood cells in the system, says Zhang. This means they have a much higher chance of interacting with and absorbing toxins, and thus can divert the toxins away from their natural targets.
In animal tests, the researchers showed that the new therapy greatly increased the survival rate of mice given a lethal dose of one of the most potent pore-forming toxins. Liver biopsies several days following the injection revealed no damage, indicating that the nanosponges, along with the sequestered toxins, were safely digested after accumulating in the liver.
If the drug can achieve regulatory approval, says Zhang, the major application would be the treatment of bacterial infections, especially those involving antibiotic-resistant bacteria. Neutralizing bacterially produced toxins not only protects the body, but can also weaken the bacteria against the immune system, since the bacteria can no longer rely on the toxins for protection, says Zhang. This is one of the ideas behind a relatively new approach to treating antibiotic-resistant bacterial infections, called anti-virulence therapy.
Zhang says his group hopes to pursue clinical trials of the nanosponge therapy soon, and he’s optimistic about its prospects. The polymer that makes up its core is already FDA-approved, and the red blood cell membrane is safe since it is taken from the body, he says. Compared to other types of drugs, says Zhang, “I envision much less hurdles for clinical trials and approval.”
Keep Reading
Most Popular
Large language models can do jaw-dropping things. But nobody knows exactly why.
And that's a problem. Figuring it out is one of the biggest scientific puzzles of our time and a crucial step towards controlling more powerful future models.
How scientists traced a mysterious covid case back to six toilets
When wastewater surveillance turns into a hunt for a single infected individual, the ethics get tricky.
The problem with plug-in hybrids? Their drivers.
Plug-in hybrids are often sold as a transition to EVs, but new data from Europe shows we’re still underestimating the emissions they produce.
Google DeepMind’s new generative model makes Super Mario–like games from scratch
Genie learns how to control games by watching hours and hours of video. It could help train next-gen robots too.
Stay connected
Get the latest updates from
MIT Technology Review
Discover special offers, top stories, upcoming events, and more.