Nanopillar Solar Cells
Researchers at the University of California, Berkeley, have made a new kind of solar cell by growing an array of upright nanoscale pillars on aluminum foil. They make bendable solar cells by encapsulating the entire cell inside a transparent, rubbery polymer. The design, the researchers suggest, could lead to solar cells that cost less than conventional silicon photovoltaics.
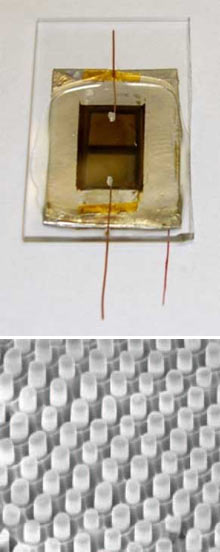
The nanopillars allow the researchers to use cheaper, lower-quality materials than those used in conventional silicon and thin-film technologies. What’s more, the technique used to make the cells could be adapted to make rolls of flexible panels on thin aluminum foil, cutting manufacturing costs, says Ali Javey, an electrical-engineering and computer-sciences professor who led the work. The work is at an early stage, and “you won’t know the cost until you do this using a roll-to-roll process,” he says. “But if you can do it, the cost could be 10 times less than what’s used to make [crystalline] silicon panels.”
The solar cells are made of uniform 500-nanometer-high pillars of cadmium sulfide embedded in a thin film of cadmium telluride. Both materials are semiconductors used in thin-film solar cells. In an online Nature Materials paper, Javey and his colleagues showed that the cells have an efficiency of about 6 percent in transforming sunlight into electricity. Others have made cells with pillar designs, he says, but they used expensive methods to grow the pillars and could not get efficiencies above 2 percent.
In conventional cells, silicon absorbs light and creates free electrons, which need to get to the electrical circuit before they get trapped at defects or impurities in the material. This requires extremely pure, expensive crystalline silicon to achieve the most efficient photovoltaic devices.
The nanopillar design splits up silicon’s duties: the material surrounding the pillars absorbs light, and the pillars transport them to the electrical circuit. This increases efficiency in two ways. The closely packed pillars trap light between them, helping the surrounding material absorb more. The electrons also have a very short distance to travel through the pillars, so there are fewer chances of their getting trapped at defects. That means you can use low-quality, less expensive materials, Javey says.
Others are making solar cells with different nanostructures. Harvard University chemistry professor Charles Lieber has made nanowires consisting of a silicon core and different concentric silicon layers. Peidong Yang, a chemistry professor at UC Berkeley, has made dye-sensitized solar cells with zinc oxide nanowires. These nanowire solar cells have reached efficiencies of 4 percent.
Javey and his colleagues make the nanopillar cell by first anodizing aluminum foil. This creates a periodic arrangement of 200-nanometer-wide pores, which act as templates for cadmium sulfide crystals to grow erect. Then comes a coating of cadmium telluride and the top electrode, a copper and gold film. They attach the cell to a glass plate or make it flexible by pouring polymer solution on top and setting it.
“This is exciting progress in integrating engineered nanomaterials with a diversity of soft substrates for fabricating flexible and foldable high-efficiency solar cells,” says Zhong Lin Wang, a materials-science and engineering professor at Georgia Tech. But the cell will have to compete with thin-film flexible solar cells made of silicon, cadmium telluride, and other materials, says Arthur Nozik, a physical chemist who studies nano solar cells at the National Renewable Energy Laboratory, in Golden, CO. As opposed to the new cell’s flexibility, he says, “I think the selling point might be low cost.”
For now, the researchers are exploring materials that could improve the cell efficiency. The top copper-gold layer, for instance, is only 50 percent transparent. If all the light falling on it went through, the cell’s efficiency could already be double, Javey says. The researchers plan to make cells with transparent conducting materials such as indium oxide. “There is significant room for improvement, at least by two times, by simply improving or replacing our top contact material,” he says.
The researchers also intend to try other semiconductor materials for the pillars and surrounding material. Javey says that the fabrication process is compatible with a wide range of semiconductors, and other combinations could up the efficiency.
Trying other semiconductor materials might also be important given cadmium’s toxicity issues, Berkeley’s Yang points out. Nevertheless, he says, “architecture is most important–materials we can continue working on. The beauty of this paper is the demonstration of how well the architecture works.”
Keep Reading
Most Popular
Large language models can do jaw-dropping things. But nobody knows exactly why.
And that's a problem. Figuring it out is one of the biggest scientific puzzles of our time and a crucial step towards controlling more powerful future models.
How scientists traced a mysterious covid case back to six toilets
When wastewater surveillance turns into a hunt for a single infected individual, the ethics get tricky.
The problem with plug-in hybrids? Their drivers.
Plug-in hybrids are often sold as a transition to EVs, but new data from Europe shows we’re still underestimating the emissions they produce.
Google DeepMind’s new generative model makes Super Mario–like games from scratch
Genie learns how to control games by watching hours and hours of video. It could help train next-gen robots too.
Stay connected
Get the latest updates from
MIT Technology Review
Discover special offers, top stories, upcoming events, and more.