Detecting Aircraft Pathogens Before It’s Too Late
Each year, an estimated 600 million passengers fly in the United States, and of those, roughly 350,000 are international travelers, according to the Bureau of Transportation Statistics. This leaves commercial airliners vulnerable to biological contamination and makes the spread of disease a real threat.
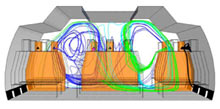
Now researchers at the MITRE Corporation have conducted a study that, for the first time, looks at the particle distribution of exhaled breath to better understand how airborne pathogens spread in aircraft cabins, and how best to detect the particles that could contain viruses.
“The most important point is that if you want to detect infectious viruses from exhaled breath, you need a biosensor with single particle detection,” says Grace Hwang, principal investigator of the study and a lead biosensors scientist at MITRE. “Most commercially available biosensors need 10 million viruses before they can inform the user that a virus of concern has been caught, and usually diagnosis takes three to four hours.” This is problematic, adds Hwang, since most viruses are found in low concentrations when expelled from an infected person, and many flights do not last more than 90 minutes.
In addition, the researchers determined that most particles stayed suspended in the aisle, so when booking a trip, take a window seat, says Michael Harkin, a member of the MITRE team, who presented the research at the 2009 IEEE Conference on Technologies for Homeland Security. Particles also did not travel far outside the contaminated row, and if they did, it was across the row. Previously, it was thought that contaminants would travel front to back, or back to front. “There was minimal exposure to the row in front of, and to the window passengers in, [the contaminated row],” says Harkin. Thus, the researchers concluded that biosensors should be placed at the ceiling of the aircraft cabin, about every four rows.
“Our goal is to capture the infected cases coming into the U.S. before people are symptomatic,” says Hwang. “That will buy time to defend against a pandemic spread, and the economic benefits would be enormous.”
The need for such sensors was evident in the 2003 outbreak of Severe Acute Respiratory Syndrome (SARS), which originated in an Air China flight from Hong Kong to Beijing, spread through 18 countries, and resulted in 774 fatalities. Asian economies suffered $11 billion in damages. “If you have an appropriate device to detect pathogens on aircraft, which is a huge challenge, then you are prepared for a deadly outbreak,” says Byron Jones, associate dean for research and graduate programs at Kansas State University, and director of its engineering experiment station. “We have seen how the swine flu spreads, and while it has turned out to be a mild disease, if it were something deadly and contagious like typhoid fever, it could be a different story.” Jones is also part of a team of experts at the Air Transportation Center for Excellence currently looking into the healthfulness of aircrafts.
The first case of H1N1 occurred in April, and since then, there have been close to 80,000 flights within North America and only one air-travel case under investigation. The risk of catching H1N1 via airliners is low but present, adds Mark Gendreau, a senior staff physician and vice chair of emergency medicine at Lahey Clinic, in Burlington, MA, and an associate professor of emergency medicine at Tufts University School of Medicine, in Boston.
To conduct the study, MITRE researchers used a computational fluid dynamics model to investigate the extreme coughing and sneezing situations of seven passengers known as “super spreaders.” (Super spreaders cough and sneeze at a rate of 50 times per hour.) The software modeled the aircraft ventilation of a Boeing 767 airliner cabin, as many prior studies have done to determine the optimal sensor placement. But that does not tell you anything about the number of particles exhaled, says Hwang. The researchers found the fluid volume in saliva and divided it by the number of particles from a sneeze and a cough to get a distribution of particles. This, coupled with the data from the computational fluid dynamics model, allowed the researchers to compute the number of collectable bioparticles, says Hwang.
The researchers found that contamination traveled farther in sneezing than in coughing cases, and that particles from the two window-seat passengers entered the outlet vents quickly and were the least circulated in the cabin. In contrast, particles from the three passengers in the center row lingered and were not transported as effectively as particles exhaled from passengers in the two aisle seats of the aircraft’s two outside rows.
For the purposes of the study, the researchers assumed that they had approximately 90 minutes to detect a virus. That’s about the length of time that it takes to fly from Vancouver to San Francisco–a flight that often carries passengers who have just arrived from Asia.
Gendreau cautions that while the study did use sophisticated modeling techniques, the researchers did make assumptions about the super spreaders: “We don’t have a good idea of super spreaders’ characteristics.” However, the Center for Disease Control is putting a lot of money into addressing such knowledge gaps, and MITRE’s study is a nice start, says Gendreau.
The MITRE researchers also determined that to detect the presence of viruses, ultrasensitive biosensors are necessary. “The particles are small and dispersed, so you need detection down to the single particle level,” says Harkin. Currently, there are no commercial biosensors that can do that. Hwang and researchers at the University of California, San Diego, are building a novel surface plasmon polariton biosensor that has performed single molecule resolution in the laboratory. The sensor uses a plasmonic substrate with a gold surface that is perforated with nanometer-wide holes. A glycoprotein is attached to the gold surface inside each hole, and the researchers monitor the resonance of the photons that get transmitted through the gold nanohole. When a pathogen like H1N1 or H1N5 binds to the glycoprotein, the resonance changes. The work was featured in Nature earlier this year.
Keep Reading
Most Popular
Large language models can do jaw-dropping things. But nobody knows exactly why.
And that's a problem. Figuring it out is one of the biggest scientific puzzles of our time and a crucial step towards controlling more powerful future models.
How scientists traced a mysterious covid case back to six toilets
When wastewater surveillance turns into a hunt for a single infected individual, the ethics get tricky.
The problem with plug-in hybrids? Their drivers.
Plug-in hybrids are often sold as a transition to EVs, but new data from Europe shows we’re still underestimating the emissions they produce.
Google DeepMind’s new generative model makes Super Mario–like games from scratch
Genie learns how to control games by watching hours and hours of video. It could help train next-gen robots too.
Stay connected
Get the latest updates from
MIT Technology Review
Discover special offers, top stories, upcoming events, and more.