The Troubled Hunt for the Ultimate Cell
Capturing the human embronic stem cell might change the face of medicine. But to get there, a small band of researchers and biotech firms must endure a federal funding ban and ethical controversy.
John Gearhart’s lab is closed to outsiders.
Rather than happening there, an interview brokered by a university public affairs officer takes place in a windowless lecture room in the bowels of the Johns Hopkins University School of Medicine. Outside, seedy east Baltimore vibrates with the energy of a bright spring day. Gearhart appears and takes a seat under the fluorescent lights. Time is short, and no tape recorders, please.
With reddish blond hair and a direct gaze, Gearhart speaks with excitement about the vast medical potential of the research going on in his lab. He describes the early stages of human life and an elusive cell found only in embryos. But there’s much about this conversation that’s fleeting, incomplete and evasive. Suddenly his voice turns defiant and he’s scowling deeply. He relates how he and his family have received threats, how other scientists have criticized his failure to publish and his close ties with industry. And then he is gone, sprung by the clock-conscious PR man.

If awards were given for the most intriguing, controversial, underfunded and hush-hush of scientific pursuits, the search for the human embryonic stem (ES) cell would likely sweep the categories. It’s a hunt for the tabula rasa of human cells—a cell that has the potential to give rise to any of the myriad of cell types found in the body. If this mysterious creature could be captured and grown in the lab, it might change the face of medicine, promising, among other remarkable options, the ability to grow replacement human tissue at will. The ES cell could, scientists hope, be a factory-in-a-dish that turns out cardiac muscles to patch heart attack victims, neurons to mend paralysis or pancreatic cells to battle diabetes. “It’s a treasure house of opportunity for developing fundamental knowledge and medical applications,” says Michael McClure, chief of the National Institute of Child Health and Human Development’s Reproductive Sciences Branch in Bethesda, Md.
That all sounds so promising. Why, then, is John Gearhart besieged? The answer is that these cells are found only in embryos or very immature fetuses, and pro-life forces have targeted the researchers who are hunting for ES cells, hoping to stop their science cold. In addition, the federal government has barred federal dollars for human embryo research, pushing it out of the mainstream of developmental biology. To make matters worse, human ES cells could conceivably provide a vehicle for the genetic engineering of people, and the ethical dilemmas surrounding human cloning threaten to spill over onto this field. Deprived of the federal funds that power most basic biomedical research and surrounded by fierce controversy, the hunt for the ES cell is being undertaken only “by a few brave souls,” says Colin Stewart, a colleague of McClure’s at the National Institutes of Health (NIH).
Extensive reporting by TR suggests that in the United States, those brave souls are drawn from fewer than a half-dozen research groups. There are also a few others in the United Kingdom, Australia and Singapore. Even this intensive survey may have missed some researchers, since some probably prefer to do their work in silence. “We’re constantly wondering what our competitors are doing, and even who they are,” says Gearhart, director of research at the Johns Hopkins department of gynecology and obstetrics.

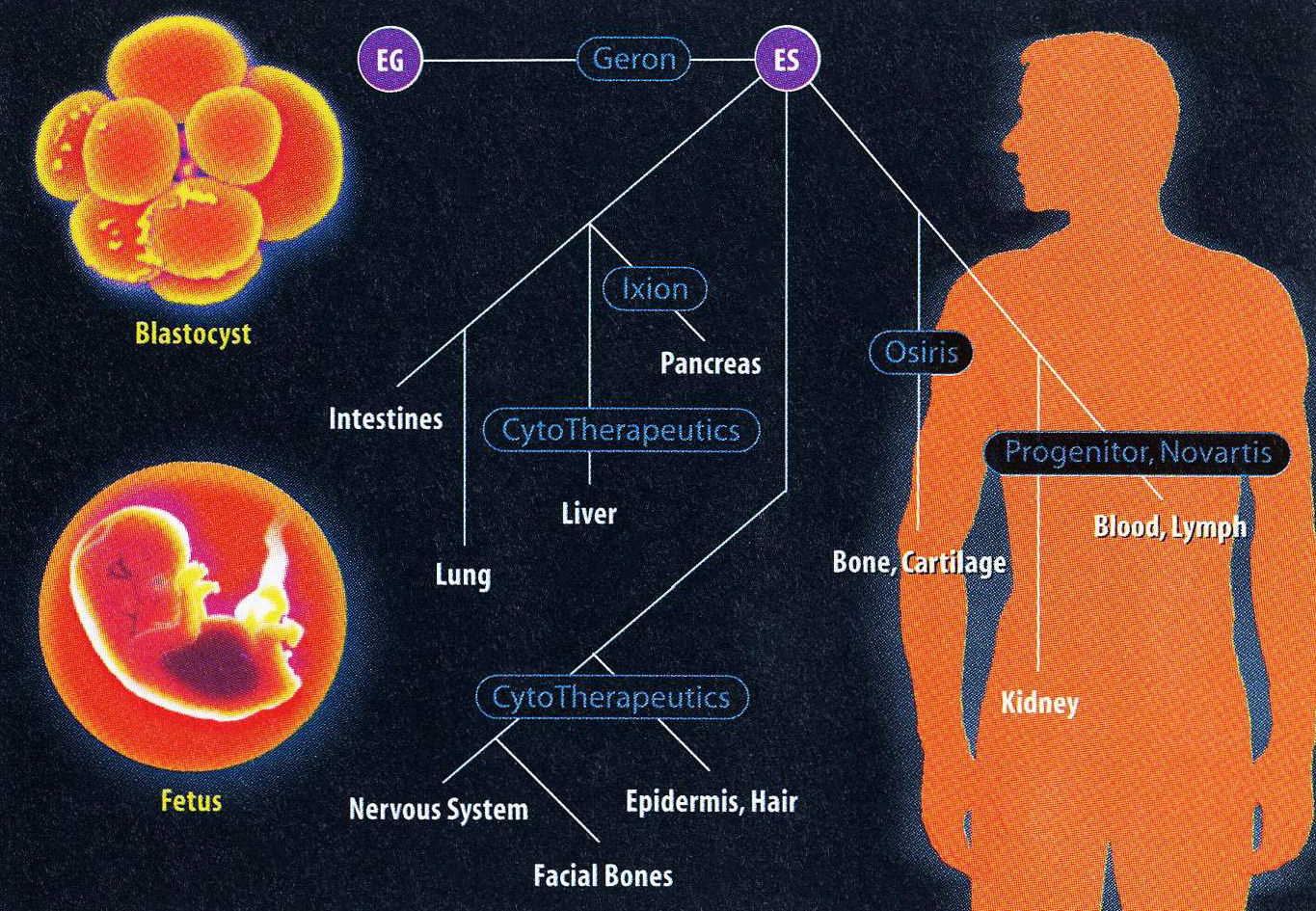
Taming the human ES cell wouldn’t just be a huge scientific coup—it would also be a potential gold mine for the biotech firm that took out an enforceable patent on the tabula rasa cell. But the same secrecy and controversy that dogs the researchers has also limited the open involvement of industry. Just one company is openly chasing the human ES cell—Geron of Menlo Park, Calif. This young Silicon Valley firm has aggressively signed collaborations with leading ES researchers, including Gearhart and Roger Pedersen, a reproductive biologist at the University of California, San Francisco (UCSF). A search of the U.S. patent filings also shows that a small startup in White Plains, N. Y., called Plurion, is building up intellectual property around the ES cell. But Plurion executive Mark Germain declines to comment further.
“It’s a taboo area,” says Doros Platika, CEO of the Cambridge, Mass.-based startup Ontogeny, a rising star in the developmental biology business. “Big pharmaceutical companies are afraid to touch it. And the field needs to sort itself out before we’d get into it.”
In spite of all these difficulties, there is a healthy scientific competition to catch the human ES cell-driven both by the desire for scientific glory and by the riches that might come with controlling the fabled stem cell itself. “It’s a race. I lose sleep,” Gearhart says. And despite many technical difficulties, several labs—including Gearhart’s—believe they may already have captured the ES cell and are working to characterize and control the cells, furiously filing patent applications as they go.
Furious scientific competition, threats of violence, huge medical potential, fear and secrecy. Welcome, behind closed doors, to the topsy-turvy world of the human embryonic stem cell.
A Breakthrough
The prize in this hunt is an invisibly small translucent dot found on the inside of an early stage of the human embryo, known as the blastocyst. Several days following fertilization, the blastocyst, a hollow ball of about 140 cells, rolls out of the fallopian tube and into the uterus, to be implanted there. Clinging to the inside of this rolling sphere are a group of identical cells-the ES cells-which are the starting point of the fetus. Soon they will divide rapidly and their descendants will take on increasingly specialized roles, emerging as heart, muscle, blood, bone, hair, nerves and all the rest of the human apparatus. For now, though, they are pure potential: holding the capacity to become any part of the body. And therein lies their mystery and their biomedical significance.
Biologists, understandably, are fascinated. But before they can study this primordial cell, they need to capture it-and control its growth-in the laboratory, something that hasn’t proved easy to do. Like physicists studying particles present at the birth of the universe by recreating its initial conditions in high-energy colliders, biologists are attempting to isolate the ES cell with a concoction of powerful biological substances that mimic those present in the first days of life.
The science behind ES cells began in earnest in 1981, when researchers in Great Britain and California independently succeeded in isolating a curious kind of cell from the interior of the mouse blastocyst. These embryonic cells were identical but each had the potential to give rise to an enormous range of different cell types-a defining mark of a stem cell.
Researchers learned how to tame the mouse ES cell in its pristine, undifferentiated state by growing it in a bed of special cells bathed in blood serum from a calf; added to the stew is a selection of proteins called growth factors. In the potion is a signal telling the ES cells not to differentiate, since their capacity to remain undifferentiated is the key to exploiting their practical potential.
Seeing the success in rodents, researchers soon began searching for ES cells in other animals, such as cows and pigs. But that turned out to be a difficult task, because the stem cells don't hang around for long. "It's in their very nature to want to become something else quickly, and it's very difficult to hold them back," says James Thomson, a biologist at the Wisconsin Regional Primate Research Center at the University of Wisconsin.
For more than a decade, the mouse ES cell stood alone. But patient, painstaking work in a number of labs slowly led to success, and reports of ES cells in other species began trickling in (see page 40).
However, by the early 1990s, no primate ES cells had been isolated. Then, in 1994, came a breakthrough. Thomson succeeded in isolating ES cells from the Rhesus macaque monkey. The discovery was a provocative hint that it might be possible to find the ES cell of another—even more interesting—primate. The race was on.
UCSF's Pedersen decided to go after the human ES cell in 1994 after becoming intrigued by his colleagues' success in growing specialized tissue, including neurons, from mouse ES cells in the laboratory. "I got fascinated with the potential" for growing transplantable human tissues, says Pedersen. He also points to a more personal motivation. When his daughter was 4 years old, a playmate of hers named Michelle Platt-Ross died of SCID—a catastrophic developmental failure of the immune system treatable only with a transplant of perfectly matched bone marrow. Like thousands of other transplant patients, Michelle had died waiting for a donor. The ES cell looked like a possible solution to those heartbreaking cases because it could—at least in theory—provide a nearly universal source of transplant tissue. "It was clear to me that if there was a any way I could help a girl like Michelle, I would grab it."
Pedersen started a low-key quest to isolate ES cells using funds from his department and studying embryos donated from UCSF's in vitro fertilization (IVF) clinic, where he serves as director of research. But soon Pedersen was looking to step up the effort. Deprived of federal funding, he accepted research backing from Geron, which had already jumped into the race by licensing rights to Thomson's Rhesus cell, and received his first check from the biotech company in early 1997.
Like everyone in this field, Pedersen isn't voluble about his work; he refuses to talk specifics about his progress. But he does note that he needs to make progress quickly, since his grant from Geron is only for two years. That kind of time frame is "quite a demanding hoop to jump through with any new technology," says Pedersen. The first obstacle was simply learning how to grow the 4-to-8 cell embryos that the group gets from the IVF lab to the blastocyst stage, where the ES cells should be found. "This is how basic the problems are," Pedersen explains. "It's not like shooting fish in a barrel."
But skill in trapping the ES cells in the lab is likely to be only one factor in deciding who wins the race. Perhaps the critical resource (and the one that effectively prevents some labs from joining the race) is access to human embryos. "There is a paucity of material available for research," explains Mark Perloe, director of Reproductive Endocrinology and Fertility at the Georgia Baptist Medical Center. "When someone is laying out $8,000 to $10,000 for IVF, it's unlikely they'll donate extra embryos to research." Instead, he says, patients generally have the extra embryos frozen for later use, or simply destroyed. "They don't want anyone else making money off their babies, and that's how they look at them—as their babies and not as a cell."
Indeed, some believe that access to healthy embryos may determine who finds the ES cell. "I would guess whoever has good stem cell experience and happens to lang next to a good IVF lab will be successful first," says Thomson. And even for those who have access, not all access is equally valuable. Thomson is hoping to repeat his Rhesus success in human but says that his collaboration with the IVF lab at the University of Wisconsin in Madison hasn't put him at the head of the pack. "Madison is a small community, and the number of embryos donated is miniscule."
Potentially, embryos could be produced and grown in the lab by simply fertilizing unwanted eggs. Although the Federal government won't fund such practices, in most states there is no law against doing it with private money. But Pedersen, for one, has ethical qualms about fertilizing eggs just for research purposes. "It might come to the time when we do it that way, but it pushed some of our own ethical buttons," he says. "We haven't wanted to cross that line yet. It's a very grey area."
Into the Fire
Back in Baltimore, Gearhart had adopted a radically different strategy-and one that appears to have propelled him to the front of the pack. He decided to sidestep the use of blastocyst-stage embryos altogether as a source of ES cells. The deciding factors were both political and scientific. The government’s funding ban, combined with the poor quality of available embryos “turned me away from that approach,” he says.
Instead, Gearhart picked up on a technique devised by cell biologist Brigid Hogan at Vanderbilt University Medical School. In 1992, Hogan showed that so-called primordial germ cells from the genital ridge (terrain destined to develop into the testes or the ovaries) of a mouse fetus could be grown in culture and acted much like ES cells. She hypothesized that the same approach might work in humans. Using aborted fetuses donated by patients, Hogan managed to isolate some interesting cells but wasn’t able to establish permanent cell lineages growing in culture-a key aim of ES research.
This alternative approach circumvented some of the funding and scientific difficulties of working with embryos. Yet in some ways it was a case of jumping out of the frying pan into the fire, since researchers using aborted fetuses are exposed to the same risk of violence from anti-abortion activists that abortion clinics face. “The threat to people working with fetal material is very real,” says Hogan.
Nevertheless, Gearhart took this strategy and ran with it—possibly all the way to the finish line. In July 1997, at the 13th International Congress of Developmental Biology in Snowbird, Utah, which was still abuzz from Ian Wilmut’s announcement that February that he had cloned a sheep named Dolly, Gearhart told a special ethics forum that he and postdoc Michael Shamblott had been growing “ES-like cells” in their lab for the preceding six months.
The connection between the ES cells and Dolly was more than just a coincidence of timing: Human ES cells could, in principle, be the vehicle for creating new breeds of human beings, as the mouse ES cells have already been used for mice. Gearhart, however, assured some attendees that neither he nor his colleagues had any intention of producing genetically altered people. His focus, he said, is strictly on the cells’ potential for saving lives by growing replacement tissues and organs and by providing important tissues for medical research. Yet even Gearhart’s colleagues understand where the fear of this new technology comes from. “It’s so easy to imagine the bad applications, since the misuse of technology, the Frankenstein myth, is already part of the vernacular,” says Pedersen, who chaired the ethics session at Snowbird.
Is Gearhart the winner in the race for the human ES cell? That’s not an easy question to answer. He and his collaborators say they have succeeded in growing “ES-like cells” from 5-to-9-week-old fetuses and are sustaining them in cell culture. But, in keeping with the field’s atmosphere of secrecy, Gearhart’s lab hasn’t yet published its results. The difference between these fetal germ cells and ES cells may well turn out to be a bone of contention among labs in the race. Gearhart, for his part, remains confident. “For all practical purposes,” he believes, these cells and ES cells will turn out to be “equivalent.”
Whether Gearhart has already won the race behind closed doors or not, the benefits for medicine of having a winner will be very large, with the largest payoff probably coming in the area of growing replacement tissues and cells.
Thomas Okarma, director of Geron’s cell therapy programs, says replacement tissues for transplant will likely be the “big hit” for human ES cells. The first type of transplantable cell Geron hopes to make are heart cells. Okarma imagines inserting a “cassette” of genetic instructions into an ES cell that would direct it to turn down the differentiation path to heart tissue. “The cells could be injected directly into the failing part of the heart,” Okarma says. The result could shore up failing heart tissue, nursing heart-attack victims back to health or providing a stop-gap for patients waiting for the right heart for a transplant.
Although Okarma envisions “a fermenter full of cells” derived from ES cells that someday will pump out new heart tissue, he stresses that the research is at an extremely early stage. To give some sense of how early, he tells TR that he hopes that within three years Geron will be testing the heart-tissue approach, using ES cells from Rhesus monkeys transplanted into other monkeys.
But the benefits of identifying and cultivating the ES cells are not only practical; there will be substantial rewards for science as well. “In theory,” says Okarma, “we should be able to generate an infinite and stable supply of [normal] human cells.” In addition to their clear medical uses, these cells, which could be turned into particular types of tissues at will in the laboratory, would be hugely useful in research. Liver cells might be used to study drug metabolism and toxicity, while other cell types might be used to test the efficacy of drug candidates. A combination of ES cell and genetic engineering techniques could also generate many interesting cell lines. Just one example: brain neurons that quickly develop the type of amyloid plaque associated with Alzheimer’s disease, providing an invaluable system for testing potential drugs to treat the ailment.
The ES cell could also open an invaluable window on human development, partly because developmental biologists would like to know which genes tell an ES cell to differentiate into more specialized cells. The proteins coded for by such genes could turn out to be new targets for drugs, or in fact be used as drugs themselves to spur, say, the regeneration of worn-out cartilage, or even to grow back receding hair.
Although the scientists at Geron are optimistic that they will be able to deliver on these promises, not everyone shares that upbeat state of mind. In spite of the apparent recent progress, some researchers who have worked with embryonic human cells doubt biologists will learn to control their growth anytime soon. H. Ralph Snodgrass, former chief scientific officer at Geron’s Menlo Park neighbor Progenitor, says, “It’s one thing to say the cells have the capacity to differentiate into all these cell types; it’s quite another to exploit that. There are some significant hurdles.”

Snodgrass is in a position to understand the practical difficulties. In the early 1990s, Progenitor, a biotech firm that also specializes in developmental biology, worked with human blastocysts, hot on the trail of the ES cell’s close cousin-an undifferentiated version of the hematopoietic stem cell (which gives rise to the full complement of cells in human blood). But Snodgrass recalls that Progenitor’s scientists couldn’t control the embryonic cell on anything other than an experimental scale-developing an actual therapy that could withstand the scrutiny of the Food and Drug Administration seemed out of the question. Progenitor has largely dropped that effort, and now focuses on better understanding the genes that control the development process in mouse embryos.
Even those who aren’t quite as skeptical as Snodgrass point out that there may be an easier route to finding a cell that could be useful as a source of replacement tissue. The shortcut involves stem cells that have already changed into a cell family, say bone or nerve, but have not yet given rise to a specific type of cell. These stem cells are a step further down the differentiation tree from the embryonic stem cell. And many scientists believe they could be far easier to isolate (partly because they are still present in adults) and nearly as useful as a source of tissue for therapies involving replacement tissues.
With so many uncertainties and questions remaining, no one is ready to declare the race for the human ES cell over or predict the winner. And it could take years to sort out the competition. Proving one has the ES cell, or even an “ES-like” cell, is no easy task since no one is exactly sure what it should look like.
According to James Robl, a biologist at the University of Massachusetts who recently saw presentations from several ES research groups at a meeting in Australia, "The cells that I have seen don't look pretty, and they don't look like ES cells. But we'll just have to wait and see." Gearhart, for one, says that he's hard at work amassing publishable and patentable proof of his cell's powers. He is pushing to show that the cells can form most or all human tissues. The first test, says Gearhart, is to grow the cells in a dish and see what they make. ES cells from mice readily form blood and even beating heart cells. Gearhart says he's seen similar tissues from his human cultures.
But the ultimate test of an ES cell’s power, says Gearhart, “won’t be done.” As in mice, that ultimate proof involves implanting human ES cells in a developing embryo, producing a germ-line chimera: a person that could pass the traits of the implanted ES cell to its own offspring. Deprived of this ultimate assay, which lies far outside the bounds of what’s ethical or even feasible, it will be impossible to meet the strictest definition of an ES cell. But, when Gearhart looks at the composite picture provided by the other tests, he says, “We’re convinced.”
But convincing the establishment of mainstream developmental biologists will take some doing. Indeed, even getting other top scientists to publicly consider the evidence may be difficult. In a glaring example of the silence enforced on researchers like Gearhart, the world's leading ES cell researchers descended this May on the University of Wisconsin for an NIH-sponsored workshop. The first of its kind, the conference featured ES specialists workin on the whole animal kingdom—from monkeys to cows. But the human ES cell was just too hot to handle. No presentations on human ES cells were allowed.
Speaking from Scotland days before the start of the conference, Austin Smith, an ES cell hunter at the Centre for Genome Research at the University of Edinburgh, said, "I understood it was to be a meeting about the human ES cell and prospects thereof." That's why Smith agreed to deliver the keynote address, and why he recommended that Gearhart and other human ES cell researchers be asked to speak. "Then they [the NIH] called me to say they couldn't invite these people. So it's officially not about human ES cells."
But Smith predicted that human ES cells would be a subject of intense—if unsanctioned—discussion. In restaurants, bars, and in hallways...everywhere but in the official sessions themselves. And that is just where the field of human embryonic stem cells stands: largely excluded from public view, but in private, a subject that is hot and getting hotter. It's only a matter of time before it bursts out from behind closed doors and begins to transform the public debate over biomedical ethics and, perhaps, much of medicine as well.
SIDEBAR 1

Ethics and Embryos
What do you get when you put an ethicist, a Methodist minister, a futurist, a theologian and a Judaic scholar together? In this case, the answer isn't a punchline: it's Geron's newly formed ethics advisory board.
Biotech companies often hire scientific advisory boards to help them anticipate and overcome technological challenges. But Menlo Park, Calif. -based Geron is leading the push to isolate the human embryonic stem (ES) cell and hopes an ethics panel will help it stave off public opposition to this controversial research. Arthur Caplan, director of the Center for Bioethics at the University of Pennsylvania Medical Center, thinks Geron is on the right track. "If they [proceed] without this discussion they are going to have to make their products behind very thick walls and in very deep bunkers," he says.
The main source of controversy is that ES research usually involves the use of human embryos, which pro-life groups vehemently oppose. As a result of the pressure, there's been a de facto ban on federal funding of such work since 1978. The climate has scared off all but a few of the best scientists, says Ronald Green, director of the Ethics Institute at Dartmouth College and professor of religion. But instead of stopping embryo experiments, the funding ban has simply curtailed federal oversight, according to Green."You create an environment where people work without scrutiny.” he says.
ES researchers working with private funds, such as Roger Pedersen at the University of California, San Francisco, who's funded by Geron, have had to find their own way through the ethical maze. For Pedersen, using extra embryos donated from in vitro fertilization clinics is acceptable, but creating new embryos specifically for the purpose of research isn't.
But many other researchers aren't willing to walk this fine line, especially when their NIH funding could be at stake. Last year Mark Hughes, a senior Georgetown University geneticist, lost his NIH grants and his job after using government-funded postdocs and equipment to perform routine diagnostic tests on embryos. Pedersen says he must now be extremely careful to maintain an "extreme and absolute" separation between his ES work and NIH-funded research projects. The divide affects everything from chemicals to staff, Pedersen says.
In addition to the embryo debate, Geron's ethics team will have to confront public fears that the potent ES cells might be misused to create designer humans or bizarre human-animal combinations. Last December, longtime biotech critic Jeremy Rifkin filed a patent laying claim to methods for making chimeras by implanting human ES cells into animal embryos. Rifkin hopes to block any Dr. Moreau seeking to profit from such hybrids.
A far-out idea? Not necessarily. Scientists already insert human genes-even whole chromosomes-into animals to make useful research species. Rifkin's patent is partly a PR stunt. He wants society to address the question, "How far can we humanize other creatures and then claim them as intellectual property?"
For Rifkin, the biotech revolution is troubling because it is being waged in an industrial setting where life is subjected to engineering rather than ethical principles. By appointing an ethics board, Geron is hoping that the two sometimes competing perspectives can be reconciled. And while it's a complex and often abstract kind of dialogue, the future of ES cell research-and all the remarkable possibilities that go with it-could be riding on this discussion.
SIDEBAR 2

Which comes first? The chicken, or the egg? (Or the embryonic stem cell?)
Picture a chicken's egg. Now replace it with Michael West's vision for an egg. It's low in cholesterol and doesn't need to be refrigerated. It still comes in a carton but is laid by a chicken that is resistant to both Marek's disease-a scourge that kills off entire flocks and to the Salmonella bacterium. Most important, it's packed with a drug vital to your well-being.
"We think this is the future of the poultry industry," enthuses West, CEO of Origen Therapeutics, a Palo Alto, Calif., firm that he founded last fall with $1 million in startup funds.
These future plans are being laid thanks to the chicken version of the embryonic stem (ES) cell-a cell that can give rise to any other specialized type of tissue in the organism. Origen's academic partner James Petitte of North Carolina State University isolated the slippery chicken ES cell, and West predicts his firm will soon do the same for the ES cells from other poultry and farm animals. The cells give scientists a blank slate to easily introduce permanent genetic changes into the animals, while "knocking out" undesired traits. "We have the door open in front of us to do really sophisticated, impressive genetic alterations," says West.
Origen's vision for finer fowl includes chickens with genes to make them more meaty as well as disease-resistant. But the biggest prize lies in turning the egg into an oval pharmaceutical factory. West says the company plans to engineer chickens that lay eggs packed with protein-based drugs that can be easily harvested by drug companies. Chickens are "the most efficient protein producing machine known to man," says West, who suggests that protein-based drugs could be produced for pennies.
ES cells are not the only tool for engineering genetic changes in animals, but thanks to some key properties they are an extremely potent one. First, ES cells grow tirelessly in culture, giving researchers ample time to add or delete DNA precisely. Then, when a genetically modified ES cell is fused into a developing embryo, it will grow to form some or all of the animal's tissue; through subsequent mating, pure breeds are made.
Origen's work with poultry could be only the tip of the transgenic iceberg. Research groups recently claim to have found ES cells in species ranging from the rabbit to the Rhesus monkey (see table). Leading the charge are startup companies like Advanced Cell Technology of Worcester, Mass. The firm was founded by University of Massachusetts biologist James Robl, whose lab succeeded in capturing ES cells from the cow and the pig. Robl says the pig ES cell might be the key to using animal organs for transplant into humans. Putting pig hearts or kidneys into people hasn't worked well until now because the human immune system is acutely sensitive to certain molecules on the pig organs. Robl, however, hopes to breed pigs free of these molecules.
Origen's West likens the ES cell to a computer breadboard and says there's no limit to the number of new circuits that can be built by reshuffling genetic components among species. "There are literally millions of inventions to be made," he says.
Deep Dive
Biotechnology and health
How scientists traced a mysterious covid case back to six toilets
When wastewater surveillance turns into a hunt for a single infected individual, the ethics get tricky.
An AI-driven “factory of drugs” claims to have hit a big milestone
Insilico is part of a wave of companies betting on AI as the "next amazing revolution" in biology
The quest to legitimize longevity medicine
Longevity clinics offer a mix of services that largely cater to the wealthy. Now there’s a push to establish their work as a credible medical field.
There is a new most expensive drug in the world. Price tag: $4.25 million
But will the latest gene therapy suffer the curse of the costliest drug?
Stay connected
Get the latest updates from
MIT Technology Review
Discover special offers, top stories, upcoming events, and more.