Why Kickstarter’s Glowing Plant Left Backers in the Dark
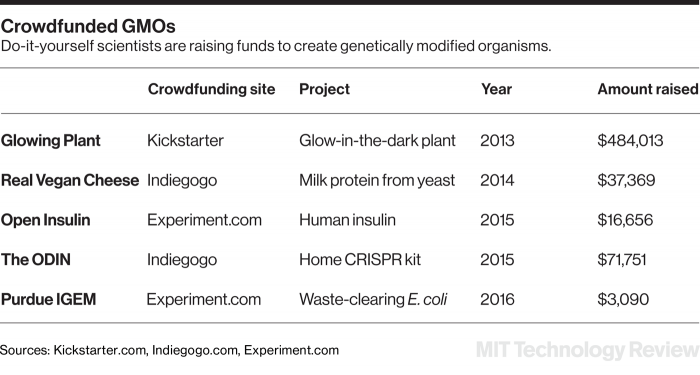
In any discussion of biohacking, Exhibit A is likely to be the “glowing plant,” the wildly successful 2013 Kickstarter campaign that raised $484,013 to create bioluminescent plants visible at night.
The project captured an idea with growing cachet: that DNA is just computer code, living things mere hardware. This view has been reinforced by the quickly falling cost of both reading the DNA molecule and synthesizing it. If biology can be managed from a computer screen, if it is de-skilled and democratized, then what follows is what the glowing-plant team calls “a world where bio-engineering is as easy and commonplace as mobile application development is today.”
Just one problem, though. There is still no glowing plant. The project, which has since morphed into the company Taxa Biotechnologies, has not made any plants that emit light unassisted. The seeds it promised to its backers are already two years overdue. “What it says is that biotechnology is not as easy as portrayed in the popular media,” says Todd Kuiken, a scholar at the Woodrow Wilson Center in Washington, D.C., who studies synthetic biology and was among the backers of the project. “All these stories that people are going to make viruses or new animals in their garage—well, it’s just not as easy as connecting Legos together.”
Do-it-yourself biology promises to let ordinary people or small teams of amateurs participate in what is already a great scientific revolution shaping the 21st century. Lately, the DIY biology movement has gravitated to “synthetic biology,” the trend in academia and industry that advocates for precise and extensive engineering of living things, genetic modification using off-the-shelf DNA “parts,” and even construction of complete life forms synthesized from raw materials.
Things reviewed
Glowing Plants: Natural Lighting with No Electricity
Created by Antony Evans on Kickstarter.com
“Synthetic Biology and Biosecurity: Challenging the ‘Myths’”
By Catherine Jefferson, Filippa Lentzos, and Claire Marris Frontiers in Public Health
August 2014
The combination of DIY biology and synthetic biology—biohacking, in other words—is so far mostly rhetorical. Yet the Kickstarter campaign certainly made it sound easy. Its creators said they would take genes from fireflies or bioluminescent bacteria and add them to a plant to make it emit a greenish light. Anyone who donated $40 was promised a plant within 12 months. For a $150 contribution, you’d get a glowing rose. The project’s core aim has been to add six genes to the genome of tobacco plants and coördinate them as an entire metabolic pathway.
But that has proved very difficult to do. “It was a poor choice of product. It’s on the edge of what’s possible,” says Antony Evans, the Cambridge University math major, former mobile-app marketer, and entrepreneur who is CEO of Taxa and heads the project. “I personally feel terrible we haven’t shipped yet. But it’s not like we took the money and ran.” Just the opposite: rather than conceding defeat, Evans this summer raised another $250,000 on Wefunder, a site that allows any member of the public to buy shares in risky private companies. It’s like a new Kickstarter, this time with stock instead of products in return. Evans says the new funding will first go to a different, more feasible product—a fragrant moss with a single added gene that makes it smell like patchouli oil—but that the team is still working on the plant.
Growing trend
The glowing-plant project is so far the highest-profile venture to come out of the do-it-yourself biology community. There are now some 86 DIY biology spaces or groups around the globe, in places including Auckland, Kansas City, and Paris, according to a tally kept by the website DIYBio.org. The grandfather of the DIY spaces is GenSpace, based in Brooklyn, a nonprofit membership group whose founder, the biologist Ellen Jorgensen, has emphasized the movement’s educational purpose, saying that “the best way to understand biotechnology is to experience it through practice.”
The DIY movement is mostly hobbyists and educators, but increasingly it also has the ambition to create medicines or new consumer products outside of big companies or academia. Projects under way include an effort to produce milk protein in modified yeast cells so it can be used for cheese making. Another team is attempting to make human insulin in bacteria, reinventing something first achieved by the biotechnology industry in 1978.
The significance of such independent undertakings was described by physicist Freeman Dyson in “Our Biotech Future,” an influential 2007 essay in the New York Review of Books. Dyson argued that the trend embodied by digital cameras, personal computers, and GPS receivers would “soon be extended from physical technology to biotechnology.” He also anticipated how home-brew projects would derive their importance from their ethical thrust—insulin free of drug-company profits, cheese without dairy cows, light without electricity. Instead of Monsanto with its labs full of experts striving for the next pesticidal seed, a new “domestic” biotechnology would be oriented toward green technology with utopian aims.
The Kickstarter campaign for a glowing plant succeeded because it tapped into these aspirations. “It was the first big synthetic-biology project that had ever been crowdfunded,” says Maria Chavez, director of community engagement with BioCurious, a DIY lab in Silicon Valley. Evans understood that his group was selling not just a plant but a vision that, much as 3-D printers had done, promised new sources of invention, creativity, production—and profits. If DNA was really just code, then biohacking could be seen as a modern version of the Homebrew Computer Club, the 1970s hobbyist group that spawned the first Apple computer and, eventually, the world’s largest company.
What the team didn’t budget for was how hard engineering the plants would be. They knew a dimly glowing tobacco plant had been made before, in 2010, but the scientist who carried out that work, Alexander Krichevsky, says it took him three years leading a lab at a well-equipped university, SUNY Stony Brook, to do it. Krichevsky has since started his own glowing-plant company, Bioglow, and says he has spent another three years trying to make the plants bright enough to interest consumers, a task that is ongoing. He says it was obvious to anyone in plant biology that Taxa’s time lines were unrealistic. “I was surprised by the promises they made. I thought, maybe they know something I don’t. Now I see that it is delusional,” he says. “They didn’t deliver anything for three years, and I strongly doubt they ever will.”
Tacit knowledge
Indeed, the “supposed trend” toward greater ease of access to biological engineering is exaggerated, says Claire Marris, a sociologist of science at City University London. That misperception, she says, has led to unfounded fears over home-brew garage bioterrorism, such as when the European Commission in 2007 warned of the possibility of “à la carte” viruses and microbes. In fact, Marris says, engineering actual products, like terror germs, is so difficult that such concerns are not realistic. She points out that biological work, rather than being scripted, is heavily influenced by “tacit knowledge,” like the secrets of a chef that don’t appear anywhere in a written recipe.
The glowing-plant team did manage to carry out many scientific steps without touching a petri dish. Gene sequences were designed on computers and fetched by mail order from distant supply houses. The strands were even fused into a longer genetic program using a rent-a-lab operated by other entrepreneurs. Yet while it was possible to script a bioluminescent program, instantiating it inside a plant is much harder. The organism doesn’t want to waste its energy on an extraneous process like glowing, and it is liable to undertake complex countermeasures. Even if six genes are added correctly (itself a daunting challenge), the plant will furiously attempt to silence or extinguish them, says Krichevsky. That leads to a challenging scientific problem solved only through grinding trial and error.
As it proved difficult, Evans and the project’s scientific founder, Kyle Taylor, a plant science PhD from Stanford, started clashing over the project’s real purpose: was the glowing plant a potentially important new business or just a DIY demonstration? The conflict reflected the larger question facing DIY biology.
In 2014, the team made it into the first class of biotech companies to be accepted into Y Combinator, the high-profile tech accelerator that invests $120,000 in each startup and helps it polish an investor pitch—a process that has produced phenomena like Airbnb and Dropbox. Y Combinator’s organizers believed that it had become easier for small groups of entrepreneurs to build potentially significant companies in biology, opening biotech to the rapid time lines and change-the-world investor pitches so common in software. The biotech graduates of the program include Ginkgo Bioworks, the Boston synthetic-biology company that recently raised $100 million from software investors.
Evans, the entrepreneur, was probably correct that a glowing plant, inexpensively propagated in a greenhouse and sold as novelty, could make people wealthy. Some entrepreneurs had previously engineered aquarium fish to fluoresce in red, yellow, or green under a fish tank’s light, and the rumor was they had struck it rich. But the plant hackers couldn’t get rich unless the plant glowed. And Evans says he almost gave up this February. That is when Taxa tested plants into which they’d inserted a genetic cassette they were sure would work. Instead, they found the plants didn’t emit any light at all. They were duds. It appears one of the genes had broken when it was fired into the plant. “That was the first time I started to have doubts about whether we would ever get there,” says Evans.
As for Taylor, he says he always saw the point as inspiring people to get interested in science, not raising more money. The Kickstarter campaign “was an interesting case where you could have a voyeuristic look over my shoulder, to show what goes on in a lab is pain, hard work, and failure,” he says. “I viewed it as educational. But that isn’t the way it was viewed by others.” By the time of the campaign, he’d also concluded that getting a plant to glow in a way that is visible to the naked eye was going to be difficult. “As I dug in more, the depth of the problem became much more apparent. I started to understand what it would take to become an actual product,” he says. He resigned from the project in 2015 and now says, “I am trying to put the glowing plant behind me.”
For now, there are still lots of true believers in Taxa and synthetic biology, and by many accounts the project succeeded as an educational venture. Evans has posted more than 60 updates, offering blow-by-blow details of the team’s efforts and struggles. Josh Melnick, a student from Ohio with a master’s in microelectronic engineering, wrote to tell me he has “been taken for $250” by the project. Yet he says it inspired him to start studying genetic engineering, and he’s begun hanging around DIY biology labs. “I have fallen under the allure of synthetic biology,” says Melnick, who dreams of making a new living thing all his own. Whatever he makes, he expects that it will probably be more of an art project than a product.
This story was updated on August 23, 2016.
Deep Dive
Biotechnology and health
How scientists traced a mysterious covid case back to six toilets
When wastewater surveillance turns into a hunt for a single infected individual, the ethics get tricky.
An AI-driven “factory of drugs” claims to have hit a big milestone
Insilico is part of a wave of companies betting on AI as the "next amazing revolution" in biology
The quest to legitimize longevity medicine
Longevity clinics offer a mix of services that largely cater to the wealthy. Now there’s a push to establish their work as a credible medical field.
There is a new most expensive drug in the world. Price tag: $4.25 million
But will the latest gene therapy suffer the curse of the costliest drug?
Stay connected
Get the latest updates from
MIT Technology Review
Discover special offers, top stories, upcoming events, and more.